 |
 |
| Korean J Intern Med > Volume 37(4); 2022 > Article |
|
Abstract
Background/Aims
Little is known about the clinical characteristics and treatment outcomes of ST-segment elevation myocardial infarction (STEMI) in Korea during the coronavirus disease 2019 (COVID-19) era. We aimed to evaluate the clinical characteristics and treatment outcomes of patients with STEMI in the COVID-19 era.
Methods
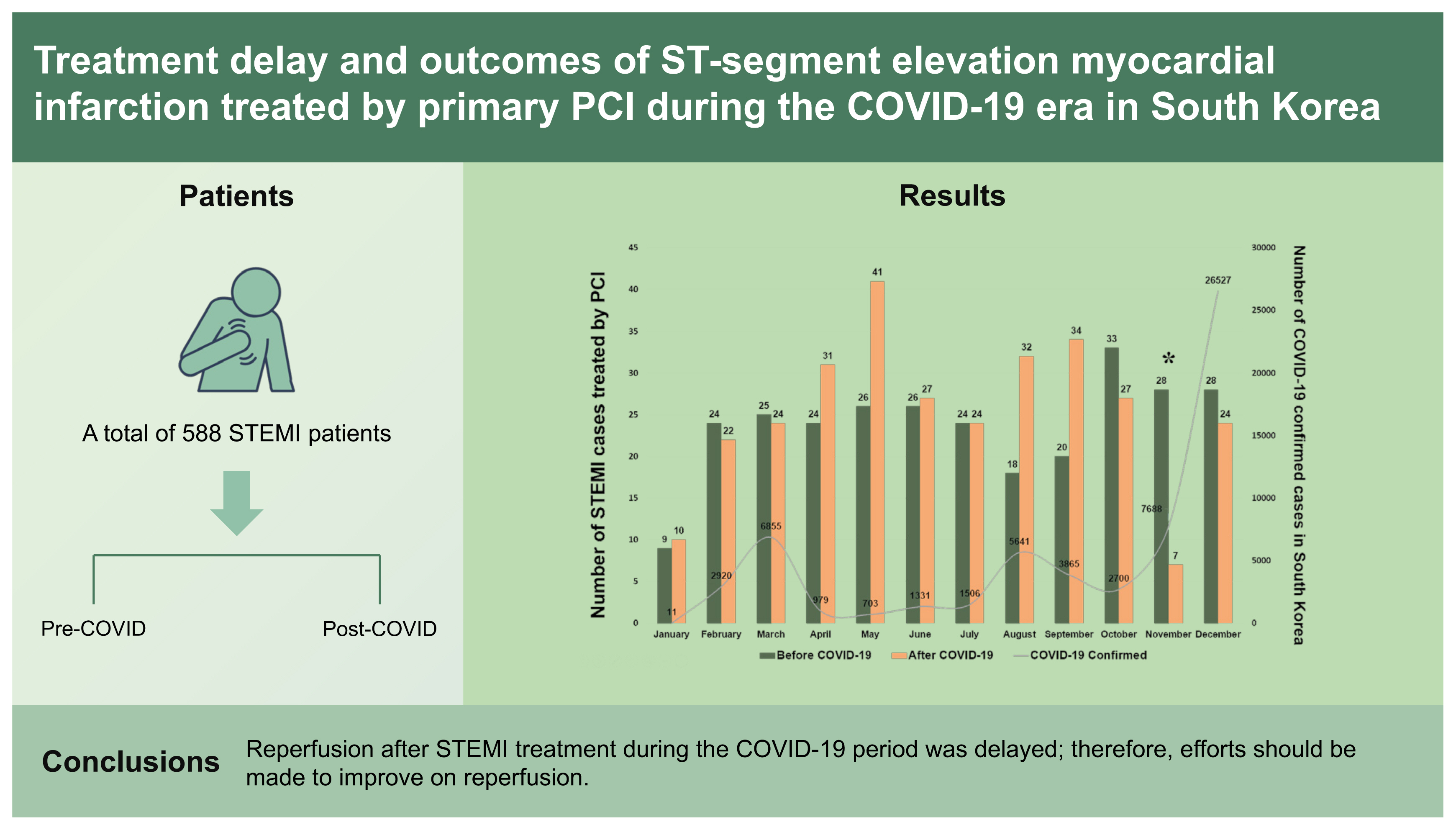
A total of 588 consecutive patients with STEMI who underwent primary percutaneous coronary intervention were included in this study. The patients were categorized into the COVID-19 (from January 20, 2020 to December 31, 2020) and control groups (from January 20, 2019 to December 31, 2019).
Results
The COVID-19 group showed pre-hospital and in-hospital delays than the control group. The control group underwent more thrombus aspiration and had a higher proportion of left main coronary artery diseases, while the COVID-19 group had a higher proportion of multivessel diseases with a marked increase in the number and total length of stents than the control group. As for the prescribed medications, the COVID-19 group was administered more beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and statins than the control group. The clinical outcomes were comparable between the groups, except for higher incidences of atrioventricular block and temporary pacemaker implantation in the COVID-19 group.
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China in December 2019 and later spread worldwide, and was declared a pandemic on March 11, 2020 by the World Health Organization [1–3]. Its outbreak is currently one of the most discussed public health issues. By August 5, 2020, 18,519,579 COVID-19 cases and 700,539 COVID-19 related deaths were reported worldwide [4]. The first COVID-19 case in South Korea was identified on January 20, 2020. By February 5, 2022, 971,018 COVID-19 cases were confirmed, with 6,858 deaths in South Korea [5].
In response to the outbreak of COVID-19, the Korean government implemented various measures, including stay-at-home orders and social distancing, which have caused a significant disruption of daily life. This affected the clinical practice environment and pattern and number of medical visits [2]. The number of emergency department (ED) visits per week declined significantly during the COVID-19 outbreak [6]. Moreover, there were significant changes in the treatment of emergency diseases, including acute myocardial infarction (AMI) and cardiac arrest [7–9]. Many clinical studies worldwide have reported a significant decline in the rates of hospitalization due to acute cardiovascular conditions and the use of catheterization laboratories [10–14]. Nevertheless, little is known about the clinical characteristics and treatment outcomes of ST-segment elevation myocardial infarction (STEMI), which requires timely and rapid percutaneous coronary intervention (PCI), in South Korea between pre-COVID-19 and COVID-19 periods. In particular, the treatment delay for STEMI was expected in the COVID-19 era, and this has been supported by clinical evidence in several studies [15–17]. Therefore, we aimed to analyze the differences in the clinical characteristics and treatment outcomes before and after the COVID-19 era in patients with STEMI in South Korea.
We included patients with STEMI who received primary PCI at the Chonnam National University Hospital (CNUH). All the participants were categorized into two groups, based on the treatment period. The participants who were admitted during the COVID-19 period were allocated to the COVID-19 group, whereas the participants who were admitted during the pre-COVID-19 period were allocated to the control group. The enrollment period of the COVID-19 group was from January 20, 2020 to December 31, 2020, whereas that of the control group was from January 20, 2019 to December 31, 2019. During this period, 602 patients were initially screened. We excluded patients who did not receive primary PCI or were finally diagnosed with non-STEMI. A total of 588 participants were eventually included. The scheme of the present study is illustrated in Fig. 1.
AMI was defined as myocardial necrosis characterized by a change (rise or fall) in cardiac markers and myocardial ischemia-related manifestations, including at least one of the following [18,19]: (1) symptoms indicative of myocardial ischemia; (2) newly found changes in the electrocardiogram indicative of myocardial ischemia; (3) the detection of pathologic Q-waves; (4) clinical evidence from cardiac imaging tools indicating loss of myocardial viability or abnormalities in the regional wall motion; and (5) the existence of angiographically confirmed intracoronary thrombus. Regarding AMI, STEMI was defined as new-onset ST-segment elevation in at least two continuous leads (> 0.2 mV in the leads V1–3 or > 0.1 mV in all the other leads on a 12-lead surface electrocardiogram), with the other components of AMI definition [18]. All the participants who were confirmed to have STEMI underwent primary PCI, which was performed by experienced medical staff, including interventional cardiologists.
During primary PCI, the imaging guidance refers to the application of intravascular ultrasound or optical coherence tomography. An infarct-related artery (or culprit artery) refers to an AMI-responsible epicardial coronary artery that was occluded or narrowed due to atherosclerotic/thrombotic process. Left main coronary artery (LMCA) disease refers to a > 50% diameter stenosis of the LMCA. Multivessel disease refers to a ≥ 70% stenosis in two or more native epicardial coronary arteries or ≥ 70% stenosis in one native epicardial coronary artery with concomitant LMCA disease. PCI was classified into four types: (1) drug-eluting stent implantation; (2) drug-coated balloon angioplasty; (3) simple balloon angioplasty; and (4) no use of stent or balloon material.
Based on the timing of hospital visits, all the participants were subdivided into two groups (on-hour and off-hour groups). On-hours included Monday–Friday 8:00 AM to 6:00 PM, whereas off-hours included Monday–Friday 6:01 PM to 7:59 AM, all weekends (Saturday and Sunday), and all non-working public holidays in South Korea [20].
In the present study, we used the following variables as the indicators of pre-hospital and in-hospital delay for primary PCI for STEMI: (1) total ischemic time (TIT); (2) symptom-to-door time (S2DT); and (3) door-to-balloon time (D2BT). The onset of symptoms was determined after interviewing the patient, and S2DT was defined as the interval from the onset of symptoms to the arrival at the ED. D2BT was defined as the interval from the time of ED arrival to when the initial ballooning was performed during the primary PCI. The TIT was the summation of the S2DT and D2BT.
In addition to timely and adequate reperfusion, all the participants received optimal medical therapies, including aspirin and P2Y12 inhibitors. Additionally, other medicaments, inclusive of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), statins, oral anticoagulants, and proton pump inhibitors, were administered to all the participants in the absence of contraindications.
For the patients discharged successfully, information on the prescribed medications (aspirin, P2Y12 inhibitors, beta-blockers, ACE inhibitors/ARBs, statins, oral anticoagulants, and proton pump inhibitors) was analyzed.
We scrutinized the clinical, angiographic, and procedural characteristics of all the survivors. Information on the prescribed medications and post-discharge treatment was collected. The baseline clinical characteristics including the sex, age, utilization of emergency medical service (EMS), timing of hospital visit (on-hours and off-hours presentation), Killip functional classification (Killip functional class I–II and III–IV), pre-hospital and in-hospital delay (TIT, S2DT, and D2BT), serum creatinine level, previous history (hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, use of dialysis, atrial fibrillation, prior myocardial infarction [MI], prior PCI, prior coronary artery bypass grafting, prior cerebrovascular accident [CVA], peripheral arterial disease), smoking status, and left ventricular ejection fraction (LVEF) were obtained. The angiographic and procedural characteristics including the anatomical site of vascular access, use of thrombus aspiration, use of image-guided PCI, anatomical site of infarct-related artery, presence of LMCA disease or multivessel disease, type of PCI, and stent profiles (average stent diameter, total length, and number of stents) were also obtained.
All clinical outcome data were obtained from the outpatient clinic at 30 days, 3 months, and 6 months after discharge from the hospital. The primary endpoint was the occurrence of major adverse cardiac and cerebrovascular events (MACCEs). MACCE was determined as the composition of all-cause death (cardiac or noncardiac death), nonfatal MI, readmission, and CVA. The secondary endpoints were all-cause death, nonfatal MI, readmission, and CVA. Readmission was defined as any hospitalization due to angina pectoris or heart failure.
In addition to the follow-up treatment outcomes, we investigated the in-hospital outcomes of all the participants, including the in-hospital death, length of stay (LOS), and in-hospital complications. The in-hospital complications included several interventional procedures (cardiopulmonary resuscitation, extracorporeal membrane oxygenation, renal replacement therapy, temporary pacemaker, and mechanical ventilation), arrhythmic findings (atrial fibrillation, atrioventricular block, and ventricular tachyarrhythmia), pericardial effusion, stent thrombosis, CVA, and bleeding complications with a bleeding academic research consortium score of ≥ 2.
The CNUH is a major tertiary cardiovascular institution located in the metropolitan city of Gwangju. After the COVID-19 outbreak, CNUH established comprehensive infection control measures to prevent nosocomial COVID-19 infections [21]. All the patients were obliged to fill in a questionnaire with questions on travel and contact history and then received a thermal scanning test before entering the hospital. All the patients with elective procedures could be hospitalized within 48 hours before admission after a negative test result for SARS-CoV-2 performed using reverse transcription-polymerase chain reaction (RT-PCR) [22]. In the ED, patients with documented fever (> 37.5°C) or any symptoms of respiratory tract infection were conveyed to the triage room for SARS-CoV-2 rapid antigen test (RAT), instead of the SARS-CoV-2 RT-PCR test. In the triage room, physicians compulsorily wore personal protective equipment (PPE), including N95 masks, face shield, gowns, gloves, boot covers, and goggles, for protection against the nosocomial COVID-19 infection.
The CNUH maintained a consistent admission policy except for only one period (from November 13, 2021 to November 30, 2021), during which healthcare workers were infected with COVID-19 [21]. During this period, lockdown measures were implemented, including the cancellation of elective procedures, outpatient clinics, and hospital admission.
Statistical analysis was conducted to scrutinize the differences in the treatment estimates between the COVID-19 group and the control group. Discrete variables are presented as numbers with percentages, while continuous variables are presented as means with standard deviations. In the comparative analysis, discrete variables were analyzed using Pearson’s chi-square test or Fisher’s two-by-two exact test, while continuous variables were analyzed using Student’s t test or Mann-Whitney test. All results were rendered statistically significant at p < 0.05.
We applied the propensity score matching (PSM) to determine whether treatment delay-related variables, such as S2DT, D2BT, and TIT, affected the clinical outcomes. With a nearest-neighbor matching algorithm and a 1:1 ratio while a caliper width of 0.01 standard deviations of the logit of the propensity score for matching, propensity scores were formed using 29 covariates in the overall study population and 36 covariates in all the survivors, except for S2DT, D2BT, and TIT. The former included the following 29 covariates: hospital visit timing (off-hours vs. on-hours presentation), sex (male vs. female), age, out-of-hospital cardiac arrest, Killip classification (Killip III–IV vs. I–II), serum creatinine level, LVEF (LVEF < 40% vs. LVEF ≥ 40%), previous history (hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, dialysis, CVA, atrial fibrillation, prior PCI, prior coronary artery bypass graft, old MI, peripheral arterial disease), infarct-related artery (LMCA or left anterior descending coronary artery vs. left circumflex coronary artery or right coronary artery), LMCA disease, multivessel disease, vascular approach, type of PCI, stent profiles (mean diameter, total length, and total number of stents), and smoking status. The latter included prescribed medications (aspirin, P2Y12 inhibitors, beta-blockers, ACE inhibitors/ARBs, statins, oral anticoagulants, and proton pump inhibitors).
In the analysis, we computed Kaplan-Meier cumulative incidence curves stratified according to before and after the COVID-19 era. Survival curves were plotted from the time of initiation of treatment and were compared using the log-rank test. At the time of the event or the final follow-up, the participants were censored. The data were analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
In the present study, 588 consecutive patients with STEMI treated with primary PCI were included in the analysis. Among them, 285 patients were included in the control group and 303 were included in the COVID-19 group. The monthly trend in the number of patients with STEMI is shown in Fig. 2. The baseline clinical characteristics are described in Table 1, demonstrating similar trends between the groups, with the exception of pre-hospital and in-hospital delay variables. Compared to the control group, the COVID-19 group was characterized by a marked prolongation of S2DT, D2BT, and TIT.
In terms of procedural characteristics (Table 2), there were more thrombus aspirations in the control group than in the COVID-19 group. The control group had a higher proportion of LMCA diseases and a lower proportion of multivessel diseases than the COVID-19 group. Regarding stent profiles, the COVID-10 group showed a marked increase in the number and total length of stents compared with the control group. After the PSM adjustment, the remaining differences were statistically balanced, except for the treatment delay (S2DT, D2BT, and TIT) variables.
At the time of discharge from the hospital, all the patients received pharmacological treatment, as summarized in Table 3. Although antithrombotic agents including aspirin, P2Y12 inhibitors, and oral anticoagulants were similarly prescribed in both groups, patients in the COVID-19 group were administered more beta-blockers, ACE inhibitors/ARBs, and statins than those in the control group. To analyze whether treatment delay affected the follow-up clinical outcomes, the variables were well balanced using PSM.
The in-hospital outcomes of all the participants are summarized in Table 4. The COVID-19 group received a more temporary pacemaker with a higher incidence of atrioventricular block than the control group. The average LOS was shorter in the COVID-19 group than in the control group with a higher incidence of LOS < 96 hours in the COVID-19 group. The incidence of in-hospital death was comparable in the groups. Even after PSM adjustment, these findings tended to be relatively consistent. The follow-up clinical outcomes of all the survivors are summarized in Table 5 and Figs. 3 and 4, showing comparable results in any treatment estimates between the COVID-19 and control groups.
In the present study, we analyzed the characteristics and treatment outcomes of patients with STEMI before and after the COVID-19 outbreak. Intriguingly, patients with STEMI who presented at the hospital during the COVID-19 period showed marked prolongation of in-hospital and pre-hospital delays than those admitted during the pre-COVID-19 period, despite similar EMS utilization rates in both groups. In terms of angiographic and procedural characteristics, patients in the control group had more LMCA diseases and underwent more thrombus aspirations, while those in the COVID-19 group had more multivessel diseases and received a higher intensity of stent implantation. Notably, the COVID-19 group received a higher intensity of medical treatment than the control group, with more prescriptions of secondary prevention medicines, including beta-blockers, ACE inhibitors, ARBs, and statins. Despite these differences, the primary and secondary endpoints were comparable between the two groups. In terms of in-hospital outcomes, a few differences were noted, including the incidence of mean LOS, LOS < 96 hours, temporary pacemaker, and atrioventricular block. Most these differences were preserved even after PSM adjustment.
It remains unclear why AMI workflow was significantly disrupted with prolonged S2DT and D2BT in the COVID-19 group. Through literature review, we identified many studies with findings in line with our study results (prolongation of pre-hospital and in-hospital delay) [15,17,23]. Although not specified in the data of the present study, the EMS process time may be prolonged during the COVID-19 pandemic era because of safety precautions and changes in basic preparatory procedure [9,24,25]. Two studies from Japan and the United States reported that pre-hospital processing time significantly increased after the COVID-19 period than the pre-COVID-19 period. A comparative study in South Korea reported changes in EMS responses [9]. In addition to the delay in the EMS response, patient-based factors of pre-hospital delay need to be considered. Some patients may be reluctant to present to the ED or delay to seek medical care for AMI because of the fear of being exposure to or infected with COVID-19 in hospitalized care facilities [6,16,26]. In other words, the belief that healthcare facilities were overcrowded with patients with COVID-19 and the fear of contracting the COVID-19 at hospitals made patients to neglect their clinical symptoms and appropriate medical care.
There are several possible explanations for the D2BT prolongation observed in the COVID-19 era. First, most patients with AMI who presented to the ED underwent SARS-CoV-2 RAT because their clinical symptoms and/or signs resembled those of COVID-19 infection. They were screened for COVID-19 infection, including a questionnaire and thermal screening, as mentioned earlier. Although most interventional cardiologists tend not to await the RAT results before referral to a catheterization laboratory for primary PCI, it is possible that these control measures may have contributed to the prolongation of the time from the ED to the catheterization laboratory. Second, all the medical staff who participated in primary PCI needed enough time to wear the PPE when dealing with suspected COVID-19 patients, as mentioned in a clinical study conducted in Singapore [27].
Considering the time-sensitive nature of STEMI, significant delays in hospital presentation and coronary revascularization can be dangerous. Delayed reperfusion may affect in-hospital mortality and additional complications including heart failure, conduction abnormalities, cardiogenic shock, and mechanical complications [28,29]. Although the present study showed a similar incidence of in-hospital death between the two groups, it is noteworthy that both atrioventricular block and implantation of a temporary pacemaker occurred more frequently in the COVID-19 group than in the control group, given their association with delayed reperfusion and conduction disturbance.
Meanwhile, we should note that the mean LOS was shorter in the COVID-19 group compared to the control group, and the proportion of patients with an LOS < 96 hours was higher in the COVID-19 group than in the control group. Possible explanations for this finding include hospital efforts to reduce the consumption of healthcare resources during the period of post-PCI hospitalization and maintain bed availability [30], patient preference for early discharge, and concern for the risk of contracting the COVID-19 within the hospital [28].
Although most studies showed a substantial decline in the rates of AMI hospitalization in the early COVID-19 period, the overall number of patients with STEMI did not appear to decline during the COVID-era compared with that during the pre-COVID-19 period (Fig. 2). However, an exceptionally significant decline was observed in November 2020 (28 in the control group vs. 7 in the COVID-19 group), as shown in Fig. 2 (footnote). This finding may be related to the in-hospital outbreak on November 13, 2020 [21]. Following the confirmation of COVID-19 infection, the infection control team announced a strict lockdown measure, including the closure of the ED to control the spread of the infection to all the hospital occupants and hospitalized patients. Because of the ED closure till November 28, 2020, the number of patients with STEMI in November 2020 was dramatically lower than that in November 2019. According to a French cohort-based study conducted by Mesnier et al. [31], hospital lockdown led to a profound decrease in the hospital admissions for AMI, regardless of the patients’ characteristics and the regional prevalence of COVID-19.
In South Korea, there were three major waves of the COVID-19 pandemic: (1) the first wave (January 2020 to April 2020); (2) the second wave (July 2020 to October 2020); and (3) the third wave (November 2020 to February 2021) [32]. Except for November 2020, the decline in the number of patients with STEMI in these three outbreak periods was not remarkable. Rather, the number increased significantly in April, August, and September, 2020. These findings suggest that despite the patient-based factors contributing to the aforementioned pre-hospital delay, many patients had sufficient awareness of AMI, and both the local STEMI and EMS systems were operating well.
Despite the differences in the clinical, angiographic, and procedural characteristics, the treatment estimates were similar between the two groups in this study. The COVID-19 group experienced a relative delay in reperfusion treatment with delayed TIT and had a higher proportion of multivessel diseases than the control group, which indicates a relatively poor prognosis. However, the COVID-19 group received larger proportions of beta-blockers, ACE inhibitors/ARBs, and statins, which are known secondary preventive treatments for AMI, than the control group, as shown in Table 3. Notwithstanding, these adverse effects could have been attenuated by the relatively optimal medical therapy, which may have contributed to the similar follow-up outcomes.
Although the present study provides new insights into the characteristics and treatment patterns of STEMI after the COVID-19 outbreak, it has several key limitations. First, this was a single-center, retrospective study, which causes a selection bias. Although the CNUH is a high-volume tertiary cardiovascular hospital (> 1,000 hospital beds with a 24-hour accident and emergency service) [21], the sample size of the present study was small, which means that the study is likely to have a low statistical power. Moreover, the results of our study cannot be applied to other cardiovascular institutions in different settings. For these reasons, it seems difficult to generalize the clinical characteristics to all the cardiovascular centers that treated patients with STEMI. Second, there were no variables of clinical importance in the present study. For example, because there is no detailed information on delayed EMS response, a logical explanation of the EMS-based factors contributing to the pre-hospital delay could only depend on the reviews of previously published studies. Third, the present study did not provide any information on the association with the three big waves in South Korea [32–34], although it is a kind of epidemiological investigation and trend analysis of patients of STEMI in these periods. In addition to the advent of the alpha, beta, and delta SARS-CoV-2 variants, Omicron, a new SARS-CoV-2 variant, was reported in South Africa on November 25, 2021 [35,36]. Compared with earlier waves, the fourth wave, caused by the Omicron SARS-CoV-2 variant, showed a different pattern of clinical characteristics and treatment outcomes among COVID-19 patients [36,37], changing the trajectory of the pandemic. In particular, this variant was more transmissible than the delta variant and could escape the immune system with antibodies from vaccines or previous SARS-CoV-2 infection [37], which opens a new chapter for the COVID-19 pandemic. Hence, further research is required.
Despite the belief that the COVID-19 pandemic may have undesirable effects on the treatment and management of patients with STEMI and the objective evidence for delayed reperfusion treatment of STEMI in the COVID-19 period, our clinical study demonstrated that there is no significant difference between the two groups in terms of treatment. Nevertheless, efforts should be made to reduce pre-hospital and in-hospital delays in the management of STEMI. In addition, a nationwide multicenter clinical study is needed to elucidate the clinical characteristics and treatment outcomes of STEMI in South Korea during the COVID-19 pandemic in the future.
1. Compared with the pre-coronavirus disease 2019 (pre-COVID-19) period, pre-hospital and in-hospital delays for ST-segment elevation myocardial infarction (STEMI) were observed in the COVID-19 period, as shown in many other studies worldwide.
2. Efforts should be made to shorten pre-hospital and in-hospital delays in the management of STEMI to reduce in-hospital complications.
3. A nationwide multicenter clinical study is needed to elucidate the clinical characteristics and treatment outcomes of STEMI in South Korea during the COVID-19 pandemic in the future.
Acknowledgments
The authors paid tributes to all the healthcare workers dedicated to COVID-19 treatment in South Korea.
Figure 1
Flow chart of the study participants. STEMI, ST-segment elevation myocardial infarction; ED, emergency department; COVID-19, coronavirus disease 2019; PCI, percutaneous coronary intervention; NSTEMI, non-ST-segment elevation myocardial infarction.
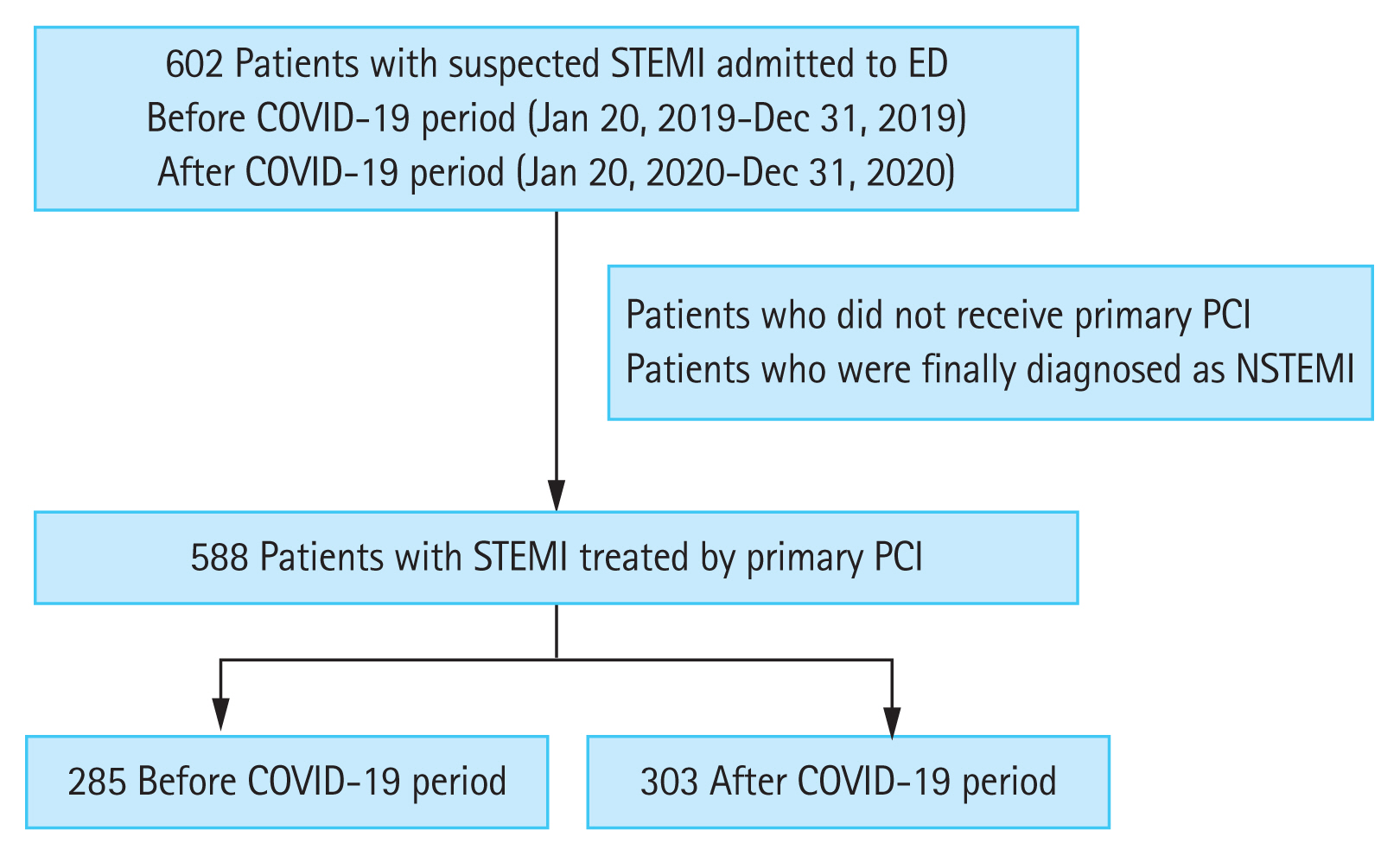
Figure 2
The monthly trend in the number of patients with ST-segment elevation myocardial infarction (STEMI) before and after the coronavirus disease 2019 (COVID-19) era. PCI, percutaneous coronary intervention. aThere was found an exceptionally significant decline in November 2020, which may be related to the in-hospital outbreak on November 13, 2020.
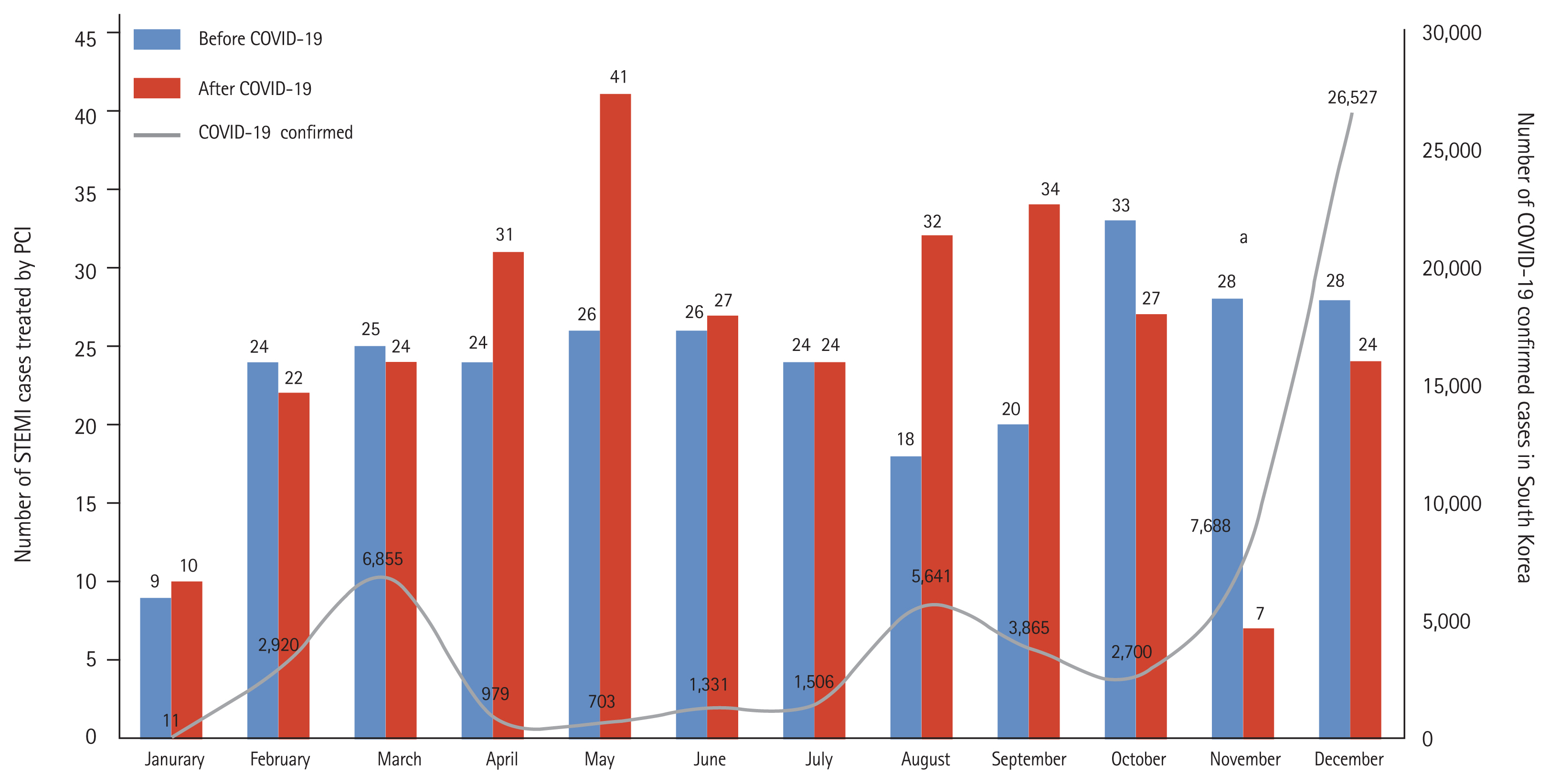
Figure 3
Kaplan-Meier cumulative incidence curves about the 6-month clinical outcomes following ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention, stratified according to before and after the coronavirus disease 2019 (COVID-19) era (before propensity score matching adjustment). (A) Major adverse cardiac and cerebrovascular event (MACCE), (B) all-cause death, (C) re-admission, (D) non-fatal myocardial infarction (MI), (E) cerebrovascular accident (CVA).
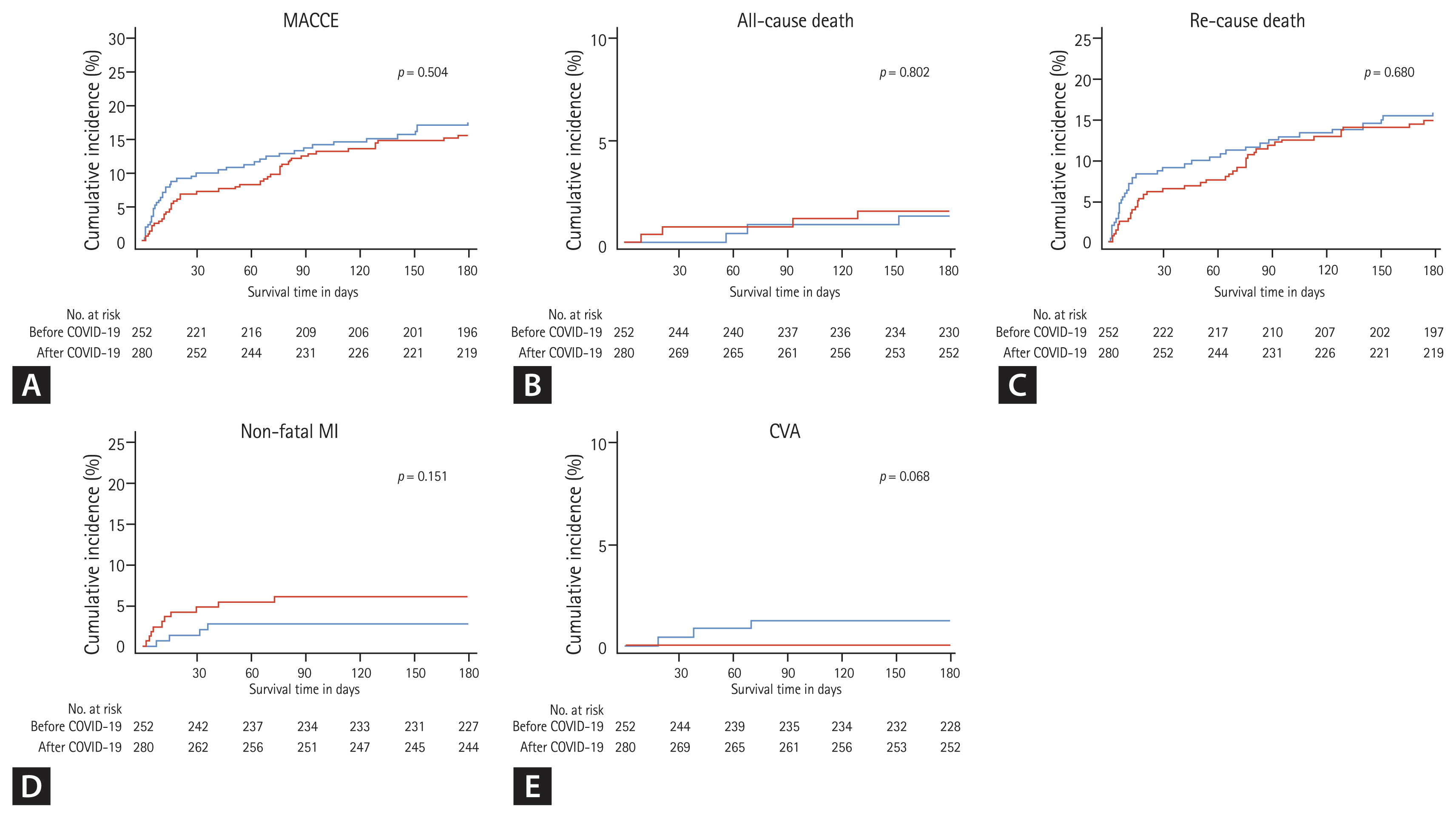
Figure 4
Kaplan-Meier cumulative incidence curves about the 6-month clinical outcomes following ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention, stratified according to before and after the coronavirus disease 2019 (COVID-19) era (after propensity score matching adjustment). (A) Major adverse cardiac and cerebrovascular event (MACCE), (B) all-cause death, (C) re-admission, (D) non-fatal myocardial infarction (MI), (E) cerebrovascular accident (CVA).
Table 1
Baseline clinical characteristics of the patients
Table 2
Angiographic and procedural characteristics of the patients
Table 3
Prescribed medications in patients who were successfully discharged from the hospital
Table 4
In-hospital outcomes of the patients
Table 5
Post-discharge treatment outcomes of patients who were successfully discharged with percutaneous coronary intervention
REFERENCES
2. Kim Y, Ahn E, Lee S, et al. Changing patterns of medical visits and factors associated with no-show in patients with rheumatoid arthritis during COVID-19 pandemic. J Korean Med Sci 2020;35:e423.




3. Sung HK, Paik JH, Lee YJ, Kang S. Impact of the COVID-19 outbreak on emergency care utilization in patients with acute myocardial infarction: a nationwide population-based study. J Korean Med Sci 2021;36:e111.




4. Dighe A, Cattarino L, Cuomo-Dannenburg G, et al. Response to COVID-19 in South Korea and implications for lifting stringent interventions. BMC Med 2020;18:321.




5. Korean Disease Control and Prevention Agency. COVID-19 [Internet] Sejong (KR): MOHW, 2022. [cited 2022 May 25]. Available from: http://ncov.mohw.go.kr/
.
6. Boserup B, McKenney M, Elkbuli A. The impact of the COVID-19 pandemic on emergency department visits and patient safety in the United States. Am J Emerg Med 2020;38:1732–1736.



7. Kwok CS, Gale CP, Kinnaird T, et al. Impact of COVID-19 on percutaneous coronary intervention for ST-elevation myocardial infarction. Heart 2020;106:1805–1811.


8. Marijon E, Karam N, Jost D, et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health 2020;5:e437–e443.



9. Lim D, Park SY, Choi B, et al. The comparison of emergency medical service responses to and outcomes of out-of-hospital cardiac arrest before and during the COVID-19 pandemic in an area of Korea. J Korean Med Sci 2021;36:e255.




10. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the COVID-19 era: incidence, clinical characteristics and in-hospital outcomes: a multicenter registry. PLoS One 2021;16:e0253524.



11. Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020;396:381–389.



12. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv 2020;13:e009438.


13. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol 2020;76:280–288.

14. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083–2088.

15. Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes 2020;13:e006631.



16. Lidin M, Lynga P, Kinch-Westerdahl A, Nymark C. Patient delay prior to care-seeking in acute myocardial infarction during the outbreak of the coronavirus SARS-CoV2 pandemic. Eur J Cardiovasc Nurs 2021;20:752–759.



17. Kobayashi S, Sakakura K, Jinnouchi H, et al. Comparison of door-to-balloon time and in-hospital outcomes in patients with ST-elevation myocardial infarction between before versus after COVID-19 pandemic. Cardiovasc Interv Ther 2022. Jan. 10. [Epub]. https://doi.org/10.1007/s12928-022-00836-4
.


18. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177.

19. Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367.

20. Oh S, Hyun DY, Cho KH, Kim JH, Jeong MH. Long-term outcomes in ST-elevation myocardial infarction patients treated according to hospital visit time. Korean J Intern Med 2022;37:605–617.




21. Lee U, Kim SE, Lee SY, et al. Source analysis and effective control of a COVID-19 outbreak in a university teaching hospital during a period of increasing community prevalence of COVID-19. J Korean Med Sci 2021;36:e179.




22. Schlosser M, Signorelli H, Gregg W, Korwek K, Sands K. COVID-19 testing processes and patient protections for resumption of elective surgery. Am J Surg 2021;221:49–52.



23. Erol MK, Kayikcioglu M, Kilickap M, et al. Treatment delays and in-hospital outcomes in acute myocardial infarction during the COVID-19 pandemic: a nationwide study. Anatol J Cardiol 2020;24:334–342.



24. Ageta K, Naito H, Yorifuji T, et al. Delay in emergency medical service transportation responsiveness during the COVID-19 pandemic in a minimally affected region. Acta Med Okayama 2020;74:513–520.

25. Satty T, Ramgopal S, Elmer J, Mosesso VN, Martin-Gill C. EMS responses and non-transports during the COVID-19 pandemic. Am J Emerg Med 2021;42:1–8.



26. Huber K, Goldstein P. COVID-19: implications for prehospital, emergency and hospital care in patients with acute coronary syndromes. Eur Heart J Acute Cardiovasc Care 2020;9:222–228.




27. Chew NW, Sia CH, Wee HL, et al. Impact of the COVID-19 pandemic on door-to-balloon time for primary percutaneous coronary intervention: results from the Singapore Western STEMI Network. Circ J 2021;85:139–149.


28. Gluckman TJ, Wilson MA, Chiu ST, et al. Case rates, treatment approaches, and outcomes in acute myocardial infarction during the coronavirus disease 2019 pandemic. JAMA Cardiol 2020;5:1419–1424.



29. Park J, Choi KH, Lee JM, et al. Prognostic implications of door-to-balloon time and onset-to-door time on mortality in patients with ST-Segment-elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Heart Assoc 2019;8:e012188.



30. Newby LK, Eisenstein EL, Califf RM, et al. Cost effectiveness of early discharge after uncomplicated acute myocardial infarction. N Engl J Med 2000;342:749–755.


31. Mesnier J, Cottin Y, Coste P, et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health 2020;5:e536–e542.



32. Kim S, Kim M, Lee S, Lee YJ. Discovering spatiotemporal patterns of COVID-19 pandemic in South Korea. Sci Rep 2021;11:24470.




33. Seong H, Hyun HJ, Yun JG, et al. Comparison of the second and third waves of the COVID-19 pandemic in South Korea: importance of early public health intervention. Int J Infect Dis 2021;104:742–745.



34. Kim SW, Kim SM, Kim YK, et al. Clinical characteristics and outcomes of COVID-19 cohort patients in Daegu Metropolitan City outbreak in 2020. J Korean Med Sci 2021;36:e12.




35. Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 2021;398:2126–2128.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



