 |
 |
| Korean J Intern Med > Volume 38(3); 2023 > Article |
|
See editorial "Significance of antinuclear antibodies in patients with COVID-19" on page 280.
Abstract
Background/Aims
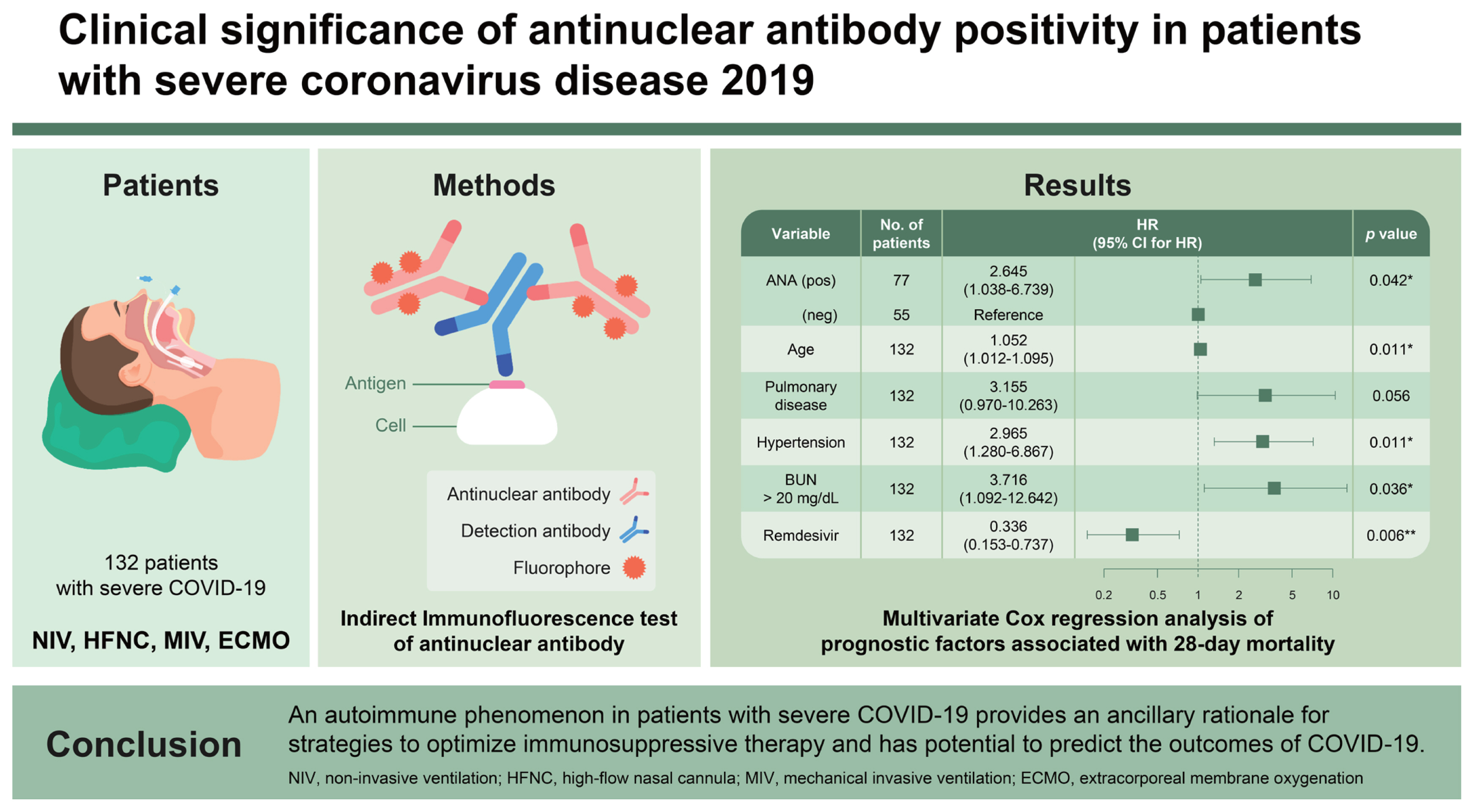
This study aimed to investigate the clinical characteristics and outcomes of fluorescent antinuclear antibody (FANA)-positive patients admitted for coronavirus disease 2019 (COVID-19) and identify FANA as a prognostic factor of mortality.
Methods
This retrospective study was conducted at a university-affiliated hospital with 1,048 beds from September 2020 to March 2022. The participants were consecutive patients who required oxygenation through a high-flow nasal cannula, non-invasive or mechanical ventilation, or extracorporeal membrane oxygenation, and conducted the FANA test within 48 hours of admission.
Results
A total of 132 patients with severe COVID-19 were included in this study, of which 77 (58.3%) had FANA-positive findings (Ōēź 1:80). FANA-positive patients were older and had higher inflammatory markers and 28-day mortality than FANA-negative patients. In the multivariate Cox proportional hazard regression analysis, FANA-positive findings (hazard ratio [HR], 2.65; 95% confidence interval [CI], 1.04ŌĆō6.74), age (per 1-year; HR, 1.05; 95% CI, 1.01ŌĆō1.10), underlying pulmonary disease (HR, 3.16; 95% CI, 0.97ŌĆō10.26), underlying hypertension (HR, 2.97; 95% CI, 1.28ŌĆō6.87), and blood urea nitrogen > 20 mg/dL (HR, 3.72; 95% CI, 1.09ŌĆō12.64) were independent predictors of 28-day mortality. Remdesivir (HR, 0.34; 95% CI, 0.15ŌĆō0.74) was found to be an independent predictor that reduced mortality.
The clinical course of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is influenced sequentially by SARS-CoV-2 itself in the replicative stage and by an aberrant host-immune response in the adaptive autoimmunity stage [1]. SARS-CoV-2 has similarities to several viruses, such as the Epstein-Barr virus, parvovirus B19, human T-lymphotropic virus-1, and human immunodeficiency virus that cause autoimmune diseases by promoting the production of pathogenic autoantibodies, such as antinuclear antibodies (ANAs), as a result of viral cross-reactivity with autoantigens [2].
Some patients with COVID-19 develop fatal complications due to a hyperinflammatory state called cytokine storm [3]. Altered T and B cell activation is associated with ANA production, which plays an essential role in tissue damage in autoimmune diseases [4]. These immune-mediated mechanisms have drawn attention to immunomodulatory therapy for patients with severe COVID-19 to attenuate viral cross-reactions with autoantigens [5]. Particularly, the current National Institute of Health (NIH) guidelines for the treatment of COVID-19 recommend immunomodulatory therapy for patients receiving oxygen therapy through a high-flow nasal cannula (HFNC), non-invasive or mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) [6]. However, the clinical usefulness of ANA as a prognostic factor for COVID-19 in critically ill patients requiring high-dose oxygen therapy has not yet been evaluated.
This study aimed to describe the clinical characteristics and outcomes of patients with COVID-19 who were fluorescent antinuclear antibody (FANA) positive and elucidate the role of FANA as a prognostic factor of mortality.
A single-center, retrospective observational study was conducted in a 1,048-bed university-affiliated hospital in the Republic of Korea between September 2020 and March 2022. The study participants included consecutive hospitalized adult patients (aged Ōēź 18 years) with reverse transcription polymerase chain reaction-confirmed SARS-CoV-2 infection who required oxygenation through an HFNC, non-invasive or mechanical ventilation, or ECMO. All patients underwent an indirect immunofluorescence test (IIFT) to detect FANA within 48 hours of admission. For patients with multiple episodes of COVID-19, only the first episode was included in our analysis. None of the study participants had a diagnosis of any autoimmune disease before SARS-CoV-2 infection.
The study protocol was approved by the Institutional Review Board of Korea University Anam Hospital (approval no. 2022AN0210), and the requirement for written informed consent was waived because this was a retrospective study.
The electronic medical records were reviewed to collect relevant demographic and clinical data: age, sex, underlying diseases, Charlson Comorbidity Index [7], COVID-19 vaccination records, laboratory findings, 28-day mortality, oxygen therapy, and use of antiviral agents or immunomodulators. Clinical severity upon admission was classified as suggested by the NIH guidelines, a division of the U.S. Department of Health and Human Services [6].
Remdesivir, dexamethasone, tocilizumab, and anticoagulants (from prophylactic to therapeutic dose) were administered according to the NIH guidelines during admission [6].
The titers and patterns of ANAs were obtained by using an automated and standardized laboratory device, EURO Pattern suite and IF Sprinter (Euroimmun AG, Leubeck, Germany). In this study, FANA with a titer of 1:80 or higher in the IIFT was assessed as positive [8]. According to the American and European Society of Rheumatology in 2019, a titer of 1:80 or higher in the IIFT was considered positive. Based on the cutoff value, patients were classified as FANA-positive or FANA-negative.
Categorical variables were classified into two groups, negative and positive. Categorical variables were presented using numbers (proportions) and compared between groups using FisherŌĆÖs exact test or PearsonŌĆÖs chi-square test, as appropriate. All laboratory values were translated into categorical variables. Continuous variables were described with median (interquartile range) values and compared between groups using the Mann-Whitney U test or two-sample StudentŌĆÖs t-test, as appropriate.
Univariate and multivariate Cox proportional hazard regression analyses were performed to select final prognostic factors for 28-day mortality. The model used backward stepwise selection. The final prognostic factors for 28-day mortality were evaluated using HarrellŌĆÖs concordance index. A HarrellŌĆÖs concordance index value close to 0.5 indicates that the model is completely random, and a value close to 1 indicates that the model fully agrees with the facts.
IBM SPSS Statistics version 23.0 (IBM Corporation, Armonk, NY, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA) were used for all statistical analyses. Two-sided p values < 0.05 were considered statistically significant.
During the study period, 132 patients with severe COVID-19 who required high-dose oxygen therapy were included. The detection rate of FANA in these patients was 58.3%: 1:80 (n = 46, 34.8%), 1:160 (n = 19, 14.4%), 1:320 (n = 9, 6.8%), 1:640 (n = 1, 0.8%), and 1:1,280 (n = 2, 1.5%); speck-led (n = 5, 3.8%), cytoplasmic (n = 5, 3.8%), mitotic (n = 2, 1.5%), nuclear (n = 10, 7.6%), homogeneous (n = 25, 18.9%), centromere (n = 1, 0.8%), and concurrent patterns (n = 29, 22.0%). Among the 132 patients, 12 with a FANA titer Ōēź 1:320 were anti-ds-DNA negative, and complement 3 or 4 was lower than the normal range in five patients. Additionally, the extractable nuclear antigen panel performed in five patients was negative.
A comparison of the demographic and clinical characteristics, and outcomes between FANA-positive and-negative patients is summarized in Table 1. Those who were FANA-positive were older than those who were FANA-negative (Table 1). Neurologic, malignant, and pulmonary diseases were more common in FANA-positive patients than FANA-negative ones (Table 1). The C-reactive protein, procalcitonin, D-dimer, and N-terminal pro-brain natriuretic peptide (NT-pro BNP) levels were significantly higher in FANA-positive patients than in FANA-negative ones (Table 1).
Remdesivir, dexamethasone, and anticoagulant were administered in 84.8%, 93.9%, and 88.6% of the 132 patients, respectively. Notably, tocilizumab was prescribed in 55 patients (41.7%). There was no difference in the major treatment modalities between the FANA-positive and FANA-negative groups (Table 1). In the FANA-positive patients, there was no significant difference in mortality between patients who received remdesivir, anticoagulant, and dexamethasone and those who additionally received tocilizumab (12/25 [48.0%] vs. 13/25 [52.0%], p = 0.492).
FANA-positive patients received mechanical ventilation or ECMO treatment more frequently than FANA-negative patients. In addition, FANA-positive patients had a significantly higher 28-day mortality rate than FANA-negative patients (Table 1).
A comparison of the demographic and clinical characteristics between survivors and non-survivors is shown in Table 2. Univariate Cox proportional hazard regression analysis using 25 variables was performed to identify the prognostic factors associated with 28-day mortality (Table 3). In the multivariate Cox proportional hazard regression analysis, 12 variables that were statistically significant in the univariate Cox proportional hazard regression analysis were selected. FANA-positive findings (hazard ratio [HR], 2.645; 95% confidence interval [CI], 1.038ŌĆō6.739), age (per 1-year; HR, 1.052; 95% CI, 1.012ŌĆō1.095), underlying pulmonary disease (HR, 3.155; 95% CI, 0.970ŌĆō10.263), underlying hypertension (HR, 2.965; 95% CI, 1.280ŌĆō6.867), and blood urea nitrogen > 20 mg/dL (HR, 3.716; 95% CI, 1.092ŌĆō12.642) were independent predictors of 28-day mortality. However, remdesivir (HR, 0.336; 95% CI, 0.153ŌĆō0.737) was found to be an independent predictor that reduced mortality (Table 4). The HarrellŌĆÖs concordance index value for prognostic factors associated with 28-day mortality in patients with confirmed SARS-CoV-2 infection was 0.825 (95% CI, 0.766ŌĆō0.884).
Our study found a FANA-positivity rate of 58.3% in patients with severe COVID-19 and discovered the usefulness of FANA as a predictor for 28-day mortality. In addition, a positive finding of FANA at the time of exacerbation tended to be significantly associated with a worse clinical course. However, no evidence was found for FANA-positive findings to be indicative of a positive therapeutic effect on immunomodulators.
Overall, our study showed a FANA positivity rate of 58.3% in patients with severe COVID-19, similar to a value of 50% reported in previous studies involving severe and critical cases of COVID-19 [9,10]. Meanwhile, the positivity rates of ANA ranged from 21.3% to 57.5% in patients with COVID-19 on various clinical spectrums [9ŌĆō14]. The differences in ANA detection rates between previous studies may be affected by disease severity and timing of sampling [9ŌĆō14]. However, previous studies did not determine the most appropriate FANA positivity threshold. ANA is not a dichotomous measure. The ANA titer can provide additional information for the diagnosis of systemic lupus erythematosus. Additionally, higher titers of ANA and the presence of other autoantibodies may have clinical implications in patients with severe COVID-19. Therefore, it may be helpful to investigate the differences in clinical characteristics according to the FANA titer.
Our findings showed that positive FANA findings were associated with older age and a higher prevalence of comorbidities such as neurologic disease, pulmonary diseases, and malignancy [9,10]. Previous findings demonstrated that a positive finding of ANA was more prevalent in older adults [15,16]. Furthermore, in previous studies, ANA was found to be positive in up to 44.4% of patients with malignancy [17,18]. The clinical significance of ANA testing in diverse neurological or pulmonary diseases is unknown. However, a previous study reported that ANA was present in 5.5% of patients with various neurological diseases, and of these, only 28% had connective tissue diseases [19]. Notably, 70% and 32% of patients with chronic obstructive pulmonary disease or interstitial lung disease were positive for ANAs, respectively [20,21]. Evidence has indicated that comorbidities are associated with the severity of COVID-19, which in turn increases mortality and morbidity [22,23].
Consistent with previous findings, we found that inflammatory markers of C-reactive protein and procalcitonin, contributing to severe COVID-19, were significantly higher in the FANA-positive group than in the FANA-negative group [10,24]. Previously, no association between ANA and inflammatory markers or D-dimer was observed [9,11,25,26]. However, in our study involving critically ill patients, D-dimer or NT-pro BNP levels were also significantly more increased in FANA-positive patients than in FANA-negative patients.
In our study, FANA-positive patients were more likely to have severe manifestations than FANA-negative patients (Table 1). Patients who were positive for ANA tended to have a severe condition and worse prognosis [11,14]. Similarly, our study identified FANA as a predictor of 28 day-mortality in critically ill patients with COVID-19. Thus, FANA detection might predict an adverse clinical course. However, further studies are needed to determine whether FANA significantly contributes to serious conditions or an epiphenomenon of severe inflammation.
This study also investigated the clinical usefulness of FANA positivity in screening patients with COVID-19 for whom immunomodulatory agents may be helpful. However, no clear benefit was observed with tocilizumab administration in patients with FANA-positive findings. In-depth analysis of the relationship between FANA-positive findings and immunomodulatory effects in a more subdivided group with a larger number of patients is needed. Interestingly, a previous report suggested that tocilizumab was only helpful when patients with severe COVID-19 showed C-reactive protein levels > 150 mg/L [27]. Therefore, it is necessary to identify the timing of administration at which the effect of the immunomodulatory agent is maximized.
Our study identified previously well-known predictors of 28-day mortality in patients with severe COVID-19. In order to reduce the disease burden of severe COVID-19, several organizations suggested that it is vital to control blood pressure [28]. Acute renal injury, an independent risk factor for in-hospital mortality, can develop in about 30% of patients with severe COVID-19 [29,30]. From this point of view, blood urea nitrogen may be considered a risk factor for mortality in patients with COVID-19 [31].
Our study has several limitations. First, this was a single-center study involving a small population. Hence, it may have a selection bias. Therefore, multicenter studies with larger sample sizes are needed to validate our findings. Second, although autoantibodies are key features of autoimmune diseases, they are not necessarily indicative of autoimmune disease. FANA can be detected transiently in acute illnesses and infections. However, no preinfection serological or long-term follow-up data were collected on the study participants. Third, our study used an ANA cut-off dilution of 1:80 as the criterion for positivity. Stratification analysis according to the FANA titer could not be performed because of the small number of study participants. Finally, laboratory findings were included as categorical variables in multivariate analysis. Therefore, our findings should be interpreted taking the cut-off values into account.
Our results suggest that severe COVID-19 is associated with a high prevalence of FANA-positive findings as an autoimmune phenomenon. This finding may support the hypothesis that optimizing immunosuppressive therapy can suppress the rapid deterioration of patients with COVID-19 resulting from immune dysfunction, such as the effects of cytokine storms. Particularly, this study suggested the potential of FANA in predicting their outcomes for COVID-19. However, in the future, additional research is needed to identify the target patients and the timing of immunomodulatory drugs by applying strategies according to the autoimmune disease characteristics of patients with COVID-19.
Notes
CRedit authorship contributions
Soo Hyun Park: conceptualization, data curation, formal analysis, methodology, project administration, visualization, writing - original draft, writing - review & editing; Jin Woong Suh: conceptualization, data curation, formal analysis; Kyung-Sook Yang: formal analysis, methodology; Jeong Yeon Kim: conceptualization, data curation; Sun Bean Kim: data curation, formal analysis; Jang Wook Sohn: conceptualization, project administration; Young Kyung Yoon: conceptualization, data curation, formal analysis, funding acquisition, writing - review & editing
Table┬Ā1
Comparison of demographic and clinical characteristics, and outcomes between the FANA-positive and -negative groups of patients with confirmed SARS-CoV-2 infection
Table┬Ā2
Comparison of demographic and clinical characteristics between survivors and non-survivors among patients with confirmed SARS-CoV-2 infection
Table┬Ā3
Univariate Cox proportional hazard regression analysis of prognostic factors associated with 28-day mortality in patients with confirmed SARS-CoV-2 infection
Table┬Ā4
Multivariate Cox regression analysis of prognostic factors associated with 28-day mortality in patients with confirmed SARS-CoV-2 infection
REFERENCES
1. Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol 2021;33:155ŌĆō162.



2. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses 2019;11:762.



3. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol 2020;11:1446.



4. Baglaenko Y, Chang NH, Johnson SR, et al. The presence of anti-nuclear antibodies alone is associated with changes in B cell activation and T follicular helper cells similar to those in systemic autoimmune rheumatic disease. Arthritis Res Ther 2018;20:264.




5. Esmaeilzadeh A, Elahi R. Immunobiology and immunotherapy of COVID-19: a clinically updated overview. J Cell Physiol 2021;236:2519ŌĆō2543.




6. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet] Bethesda (MD): National Institutes of Health, 2020. [cited 2022 Oct 26]. Available from: https://www.covid19treatmentguidelines.nih.gov/
.
7. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245ŌĆō1251.


8. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019;78:1151ŌĆō1159.

9. Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci 2020;13:1077ŌĆō1086.




10. Sacchi MC, Tamiazzo S, Stobbione P, et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci 2021;14:898ŌĆō907.




11. Lerma LA, Chaudhary A, Bryan A, Morishima C, Wener MH, Fink SL. Prevalence of autoantibody responses in acute coronavirus disease 2019 (COVID-19). J Transl Autoimmun 2020;3:100073.



12. Pascolini S, Vannini A, Deleonardi G, et al. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin Transl Sci 2021;14:502ŌĆō508.




13. Gagiannis D, Steinestel J, Hackenbroch C, et al. Clinical, serological, and histopathological similarities between severe COVID-19 and acute exacerbation of connective tissue disease-associated interstitial lung disease (CTD-ILD). Front Immunol 2020;11:587517.



14. Chang SH, Minn D, Kim YK. Autoantibodies in moderate and critical cases of COVID-19. Clin Transl Sci 2021;14:1625ŌĆō1626.




15. Xavier RM, Yamauchi Y, Nakamura M, et al. Antinuclear antibodies in healthy aging people: a prospective study. Mech Ageing Dev 1995;78:145ŌĆō154.


16. Nisihara R, Kubis MM, Rodrigues PC, Skare T, Mocelin V, Utiyama S. Antinuclear antibodies and rheumatoid factor positivity in healthy elderly adults: a cross-sectional study in 336 individuals. J Am Geriatr Soc 2013;61:2044ŌĆō2046.



17. Nisihara R, Machoski MCC, Neppel A, Maestri CA, Messias-Reason I, Skare TL. Anti-nuclear antibodies in patients with breast cancer. Clin Exp Immunol 2018;193:178ŌĆō182.




18. Gauderon A, Roux-Lombard P, Spoerl D. Antinuclear antibodies with a homogeneous and speckled immunofluorescence pattern are associated with lack of cancer while those with a nucleolar pattern with the presence of cancer. Front Med (Lausanne) 2020;7:165.



19. Michielsens B, Walravens M, Vermylen J, Carton H. Diagnostic significance of antinuclear antibodies in neurologic patients. Acta Neurol Scand 1991;84:102ŌĆō106.


20. Feghali-Bostwick CA, Gadgil AS, Otterbein LE, et al. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:156ŌĆō163.



21. Ghrairi N, Aouadi S, Elhechmi YZ, Ben Saad S, Ben Ali I, Yalaoui S. Antinuclear antibodies in interstitial lung disease: prevalence and clinical significance. Tunis Med 2019;97:1240ŌĆō1245.

22. Kim L, Garg S, OŌĆÖHalloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis 2021;72:e206ŌĆōe214.




23. Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis 2021;21:855.




24. Taeschler P, Cervia C, Zurbuchen Y, et al. Autoantibodies in COVID-19 correlate with antiviral humoral responses and distinct immune signatures. Allergy 2022;77:2415ŌĆō2430.




25. Peker BO, ┼×ener AG, Kaptan Aydo─¤mu┼¤ F. Antinuclear antibodies (ANAs) detected by indirect immunofluorescence (IIF) method in acute COVID-19 infection; future roadmap for laboratory diagnosis. J Immunol Methods 2021;499:113174.



26. Berger JS, Kunichoff D, Adhikari S, et al. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol 2020;40:2539ŌĆō2547.



27. Biran N, Ip A, Ahn J, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol 2020;2:e603ŌĆōe612.



28. Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens 2020;33:373ŌĆō374.




29. Meena P, Bhargava V, Rana DS, Bhalla AK, Gupta A. COVID-19 and the kidney: a matter of concern. Curr Med Res Pract 2020;10:165ŌĆō168.



-
METRICS

- Related articles
-
Significance of antinuclear antibodies in patients with COVID-192023 May;38(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


