 |
 |
| Korean J Intern Med > Volume 38(3); 2023 > Article |
|
Abstract
Background/Aims
Although anti-hepatitis C virus (HCV) assay is widely used to screen for HCV infection, it has a high false-positive (FP) rate in low-risk populations. We investigated the accuracy of anti-HCV signal-to-cutoff (S/CO) ratio to distinguish true-positive (TP) from FP HCV infection.
Methods
We retrospectively analyzed 77,571 patients with anti-HCV results. A receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic accuracy of anti-HCV S/CO ratio in anti-HCV positive patients.
Results
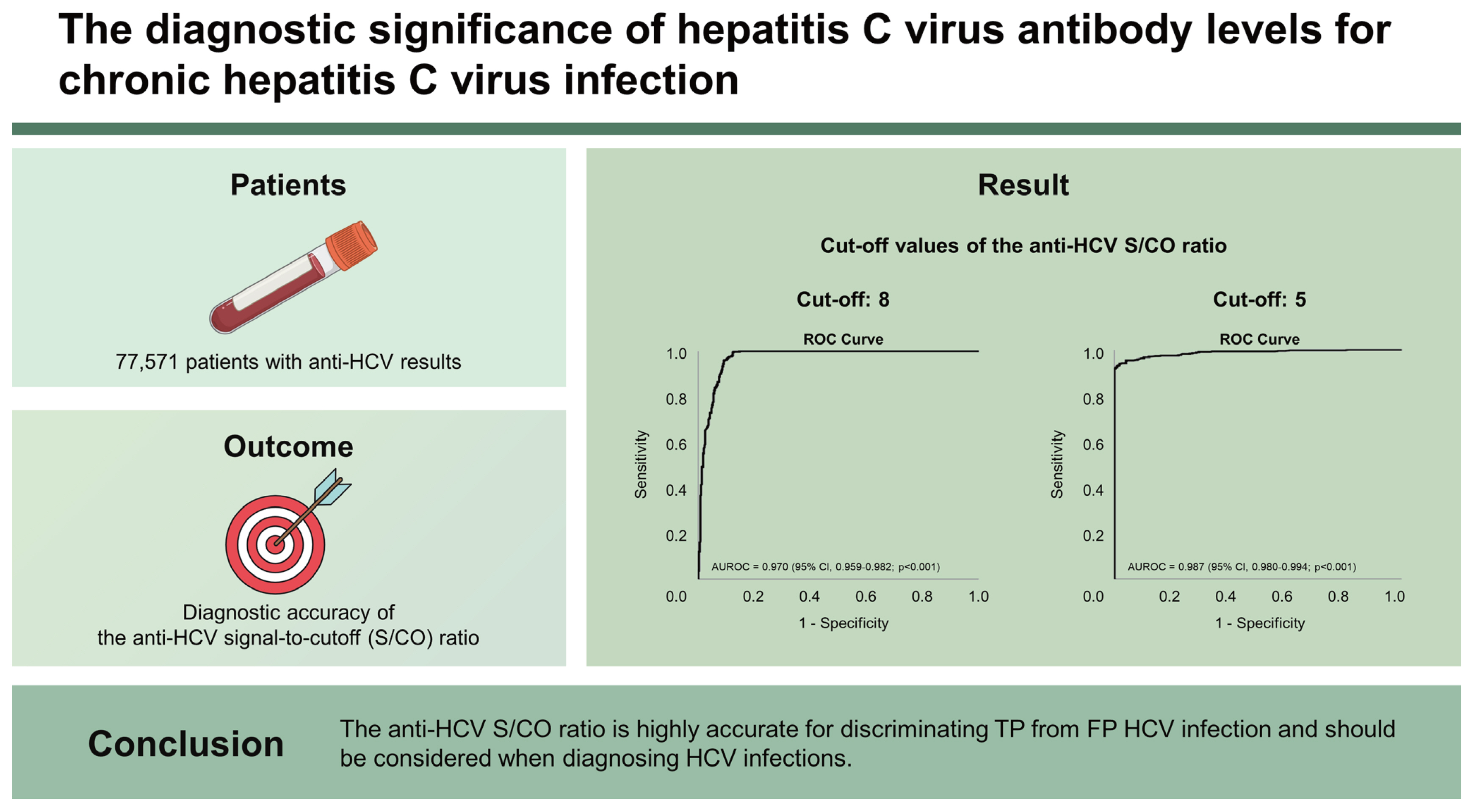
Overall, 1,126 patients tested anti-HCV positive; 34.7% of patients were FP based on HCV RNA and/or recombinant immunoblot assay (RIBA) results. The age and sex-adjusted anti-HCV prevalence was 1.22%. We identified significant differences in serum aspartate transaminase and alanine transaminase levels, anti-HCV S/CO ratio, and RIBA results between groups (viremia vs. non-viremia, TP vs. FP). Using ROC curves, the optimal cutoff values of anti-HCV S/CO ratio for HCV viremia and TP were 8 and 5, respectively. The area under the ROC curve, sensitivity, specificity, positive and negative predictive values were 0.970 (95% CI, 0.959ŌĆō0.982, p < 0.001), 99.7%, 87.5%, 87.4%, and 99.7%, respectively, for predicting HCV viremia at an anti-HCV S/CO ratio of 8 and 0.987 (95% CI, 0.980ŌĆō0.994, p < 0.001), 95.3%, 94.7%, 97.1%, and 91.4%, respectively, for TP HCV infection at an anti-HCV S/CO ratio of 5. No patients with HCV viremia had an anti-HCV S/CO ratio below 5.
The hepatitis C virus (HCV) infection remains one of the most prevalent blood-borne viral infections worldwide and is a leading cause of life-threatening liver diseases including cirrhosis and hepatocellular cancer (HCC) [1]. In 2020, the global prevalence of viremic HCV infection was 0.7%, corresponding to 56.8 million infections [2]. Based on nationwide studies in Korea, the current prevalence of HCV infection in the Korean population ranges from 0.71% to 0.78%, with prevalence increasing with age [3,4].
In 2016, the World Health Organization introduced a global goal of eliminating viral hepatitis as a major public health threat by 2030 by reducing the incidence of new chronic infections by 90% and the mortality rate by 65% [5]. The availability of highly effective direct-acting antiviral (DAA) treatment for HCV infection has made the global elimination of HCV theoretically feasible. However, the majority of people infected with HCV are not diagnosed and remain untreated [6,7], which hinders the achievement of this global goal. Consequently, there has been a change in the HCV infection management system, including scaling up screening and simplification of care and treatment services [6ŌĆō8].
The diagnosis of HCV infection is classically made based on the detection of antibodies to HCV (anti-HCV) at the first step, with confirmation on HCV RNA or HCV core antigen tests. In other words, anti-HCV is usually used as a screening test for HCV infection, and anti-HCV reactive samples require further supplemental testing for HCV RNA or HCV core antigen to definitively confirm whether there is an HCV infection with viremia. Although anti-HCV assays are highly sensitive and specific for detecting chronic HCV infection in patients [9], this test has a high false-positive (FP) rate of 15ŌĆō60% (mean, 35%), especially in low-risk populations with an anti-HCV prevalence of < 10% [10ŌĆō12]. This results in unnecessary additional tests and monitoring of the individuals who test anti-HCV positive. To overcome this problem, several studies have been conducted to reduce FP results by adjusting the signal-to-cutoff (S/CO) ratio or conducting additional tests [13ŌĆō19].
The current guidelines recommend performing reflex HCV RNA testing for all positive anti-HCV samples, but the availability of HCV RNA testing in clinical laboratories and blood banks may be limited because of its high cost and the requirement for qualified personnel and specialized equipment. Considering the easy accessibility to medical facilities and low prevalence of HCV infection, Korea is an excellent setting to evaluate whether reflex HCV RNA testing for all positive anti-HCV samples is a suitable diagnostic strategy for HCV infection.
This study aimed to determine the correct anti-HCV S/CO ratio to distinguish true-positive (TP) from FP HCV infection, estimate the true prevalence of HCV infection using the optimal anti-HCV S/CO ratio, and finally propose a suitable strategy for the diagnosis of HCV infection in low prevalence areas such as Korea.
A total of 77,571 patients with available anti-HCV results were retrospectively enrolled and analyzed at Kangdong Sacred Heart Hospital, Seoul, Korea, from January 2010 to December 2015. Individuals with HCV RNA positivity were considered as TP, viremic. Samples with a positive recombinant immunoblot assay (RIBA) result and negative HCV RNA were defined as TP, nonviremic. Samples with reactive anti-HCV screening test results but negative HCV RNA results and negative or indeterminate RIBA results were categorized as FP. These results are summarized in Table 1. Acute hepatitis C was diagnosed based on clinically acute hepatitis with no previous history of HCV and an initially low anti-HCV S/CO ratio with positive HCV RNA or documentation of anti-HCV negativity within 6 months.
The current study was conducted in compliance with the declaration of Helsinki and was approved by the Institutional Review Board (IRB) at the Kangdong Sacred Heart Hospital, Seoul, Korea (IRB number: 2022-07-017).
Routine biochemical tests were performed using standard laboratory procedures. Anti-HCV levels were determined with the ARCHITECT anti-HCV assay (Abbott Diagnostics, Wiesbaden, Germany), a chemiluminescent microparticle immunoassay for the qualitative detection of anti-HCV. According to the product reference, positivity is defined by an S/CO ratio Ōēź 1. HCV RNA testing was performed using the COBAS TaqMan Analyzer (Roche Diagnostics, Branchburg, NJ, USA; lower limit of detection, 15 IU/mL), and RIBA tests were performed using LG HCD Confirm (LG Chemical Ltd., Seoul, Korea) composed of Core 14 (core protein), Core 518 (core/NS3 fusion protein), E1E2NS4 (E1E2/NS4 fusion protein), KHCV897 (NS3 protein), and NS5 1.2 (NS5 protein) antigen.
The StudentŌĆÖs t-test for continuous variables and chi-square test for categorical variables were used for the analyses. A receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic accuracy of the anti-HCV S/CO ratio for the prediction of HCV viremia and TP HCV reactivity in anti-HCV positive patients. The anti-HCV positive rates were standardized by age and sex using the 2020 estimated population of the Korea National Statistical Office. Statistical significance was defined as a p value < 0.05. Analyses were performed using SPSS version 27.0 (SPSS Inc., Chicago, IL, USA).
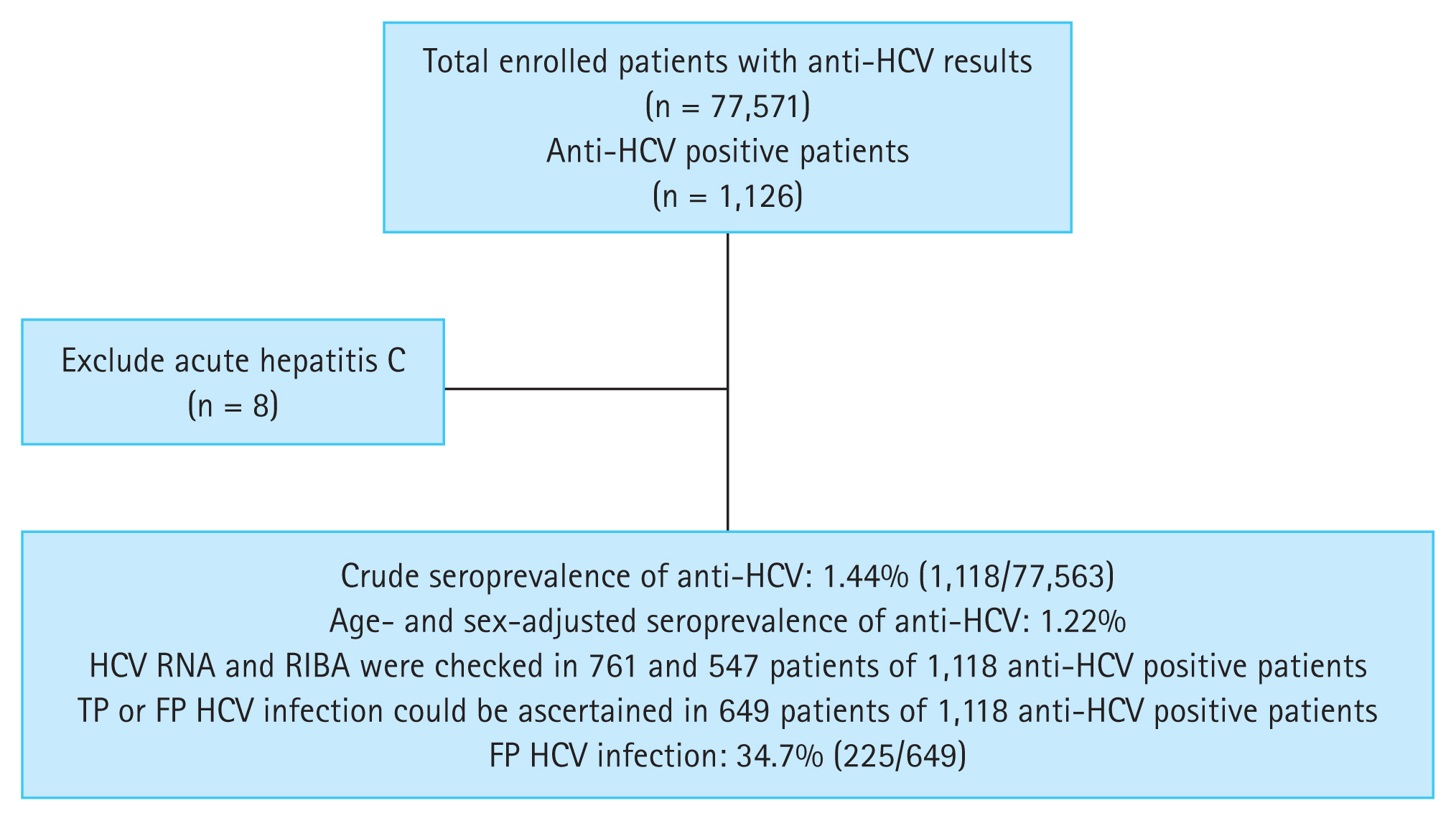
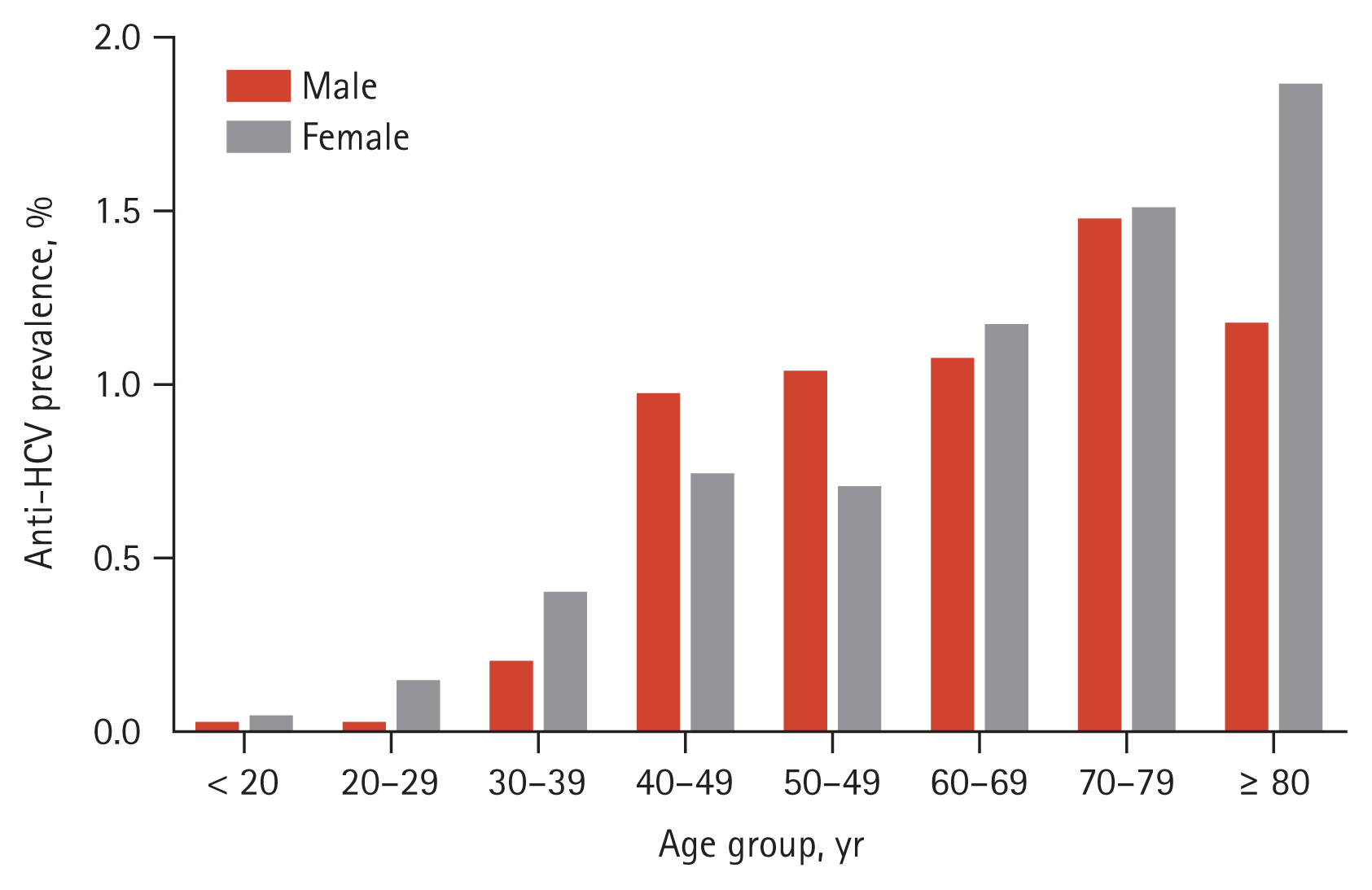
Among the 77,571 enrolled participants, 1,126 tested positive for anti-HCV. Of these, eight patients with acute hepatitis C were excluded from analysis because their serologic profiles, such as anti-HCV S/CO ratio, and RIBA results, differed from those of patients who had chronic HCV infection [20ŌĆō22] (Fig. 1). The crude seroprevalence of anti-HCV was 1.44% (1,118/77,563). The crude rate of anti-HCV in female patients (590/38,297; 1.54%) was higher than that in male patients (528/39,266; 1.34%) (p = 0.024). After adjusting for age, the anti-HCV positivity rates for female and male patients were 1.28% and 1.16%, respectively. The age- and sex-adjusted seroprevalence of anti-HCV was 1.22%. The age-specific prevalence of anti-HCV showed a gradual increase from 0.33% in subjects aged < 20 years to 3.62% in those > 80 years (Fig. 2).
Of the 1,118 anti-HCV positive patients, 528 (47.2%) were male and the mean age was 60.09 ┬▒ 17.12 years, which was significantly higher than that of anti-HCV negative subjects (48.49 ┬▒ 19.66 years, p < 0.001). HCV RNA and RIBA were measured in 761 (68.1%) and 547 (48.9%) patients, respectively. TP or FP HCV infection could be ascertained in 649 patients (58.1%) based on HCV RNA and/or RIBA results. Among them, 225 patients (34.7%) were defined as FP. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), anti-HCV S/CO ratio, and positive results of RIBA were significantly higher in the viremia group than in the non-viremia group (Table 2). The TP group showed significantly higher AST, ALT, anti-HCV S/CO ratio, and positive results of RIBA compared to the FP group (Table 3).
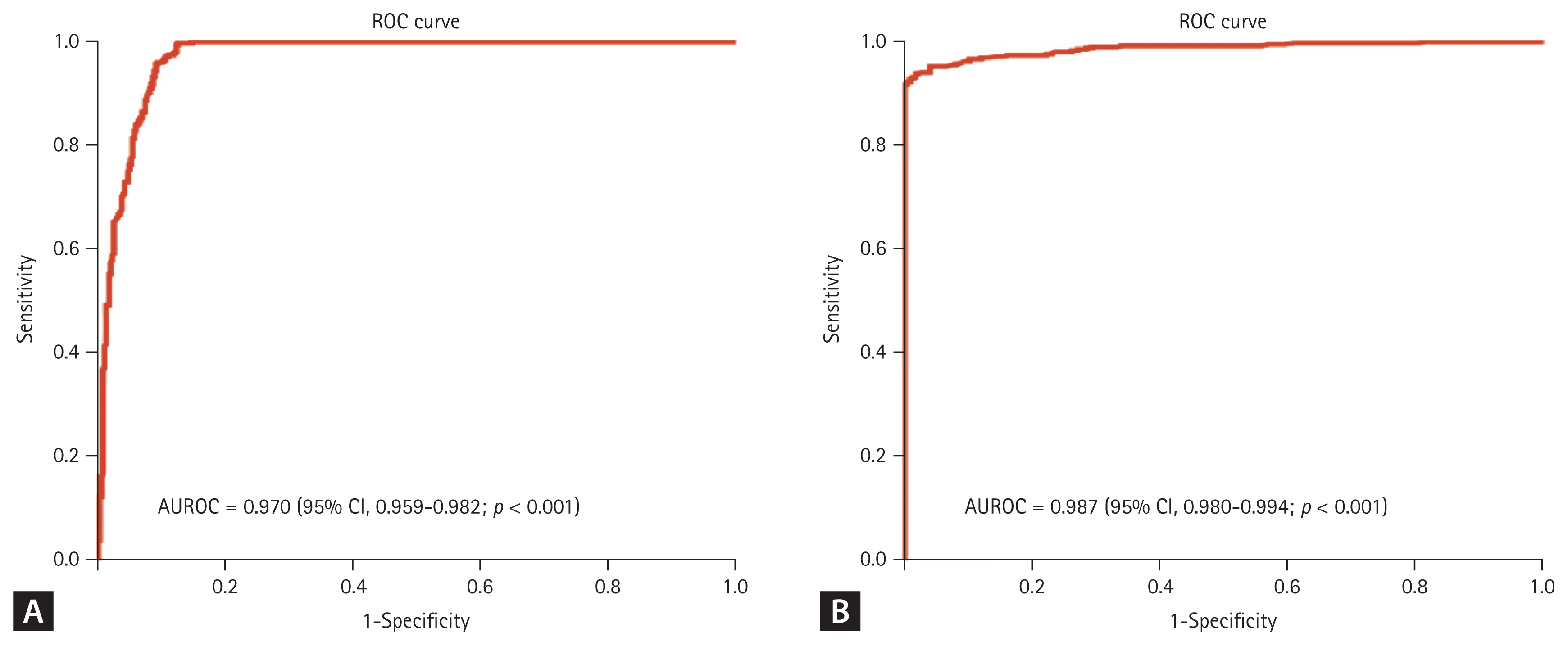
ROC curves were plotted to determine the optimal cutoff values for the differentiation of the HCV viremia group from the HCV non-viremia group, as well as TP HCV infection from FP HCV infection (Fig. 3). Figure 3A shows that the area under the ROC (AUROC) curve of the anti-HCV S/CO ratio for differentiation of the HCV viremia group from the HCV non-viremia group was 0.970 (95% confidence intervals [CI], 0.959ŌĆō0.982, p < 0.001). The best cutoff value for the anti-HCV S/CO ratio to predict HCV viremia using YoudenŌĆÖs index was 8, which yielded a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 99.7%, 87.5%, 87.4%, and 99.7%, respectively (Table 4). Regarding TP HCV infection, Figure 3B shows that the AUROC of the anti-HCV S/CO ratio for the differentiation of TP HCV infection from FP HCV infection was 0.987 (95% CI, 0.980ŌĆō0.994, p < 0.001). The optimal cutoff value of the anti-HCV S/CO ratio for TP HCV infection was 5, which yielded a sensitivity, specificity, PPV, and NPV of 95.3%, 94.7%, 97.1%, and 91.4%, respectively (Table 4).
We classified 1,118 patients into three groups comprising 509, 63, and 546 patients, respectively, using anti-HCV S/CO ratio cutoffs of 5 and 8. The relationships between anti-HCV S/CO ratio and RIBA results, HCV RNA, TP HCV infection are shown in Table 5. All patients with an anti-HCV S/CO ratio of < 5 were negative for HCV RNA. Twenty out of 240 (8.3%) and 20 out of 233 (8.6%) patients showed RIBA reactivity and TP HCV infection, respectively. Among the patients with an anti-HCV S/CO ratio of Ōēź 8, all patients showed TP HCV infection. Further, 353 out of 404 (87.4%) and 273 out of 280 (97.5%) patients showed HCV RNA positivity and RIBA reactivity, respectively.
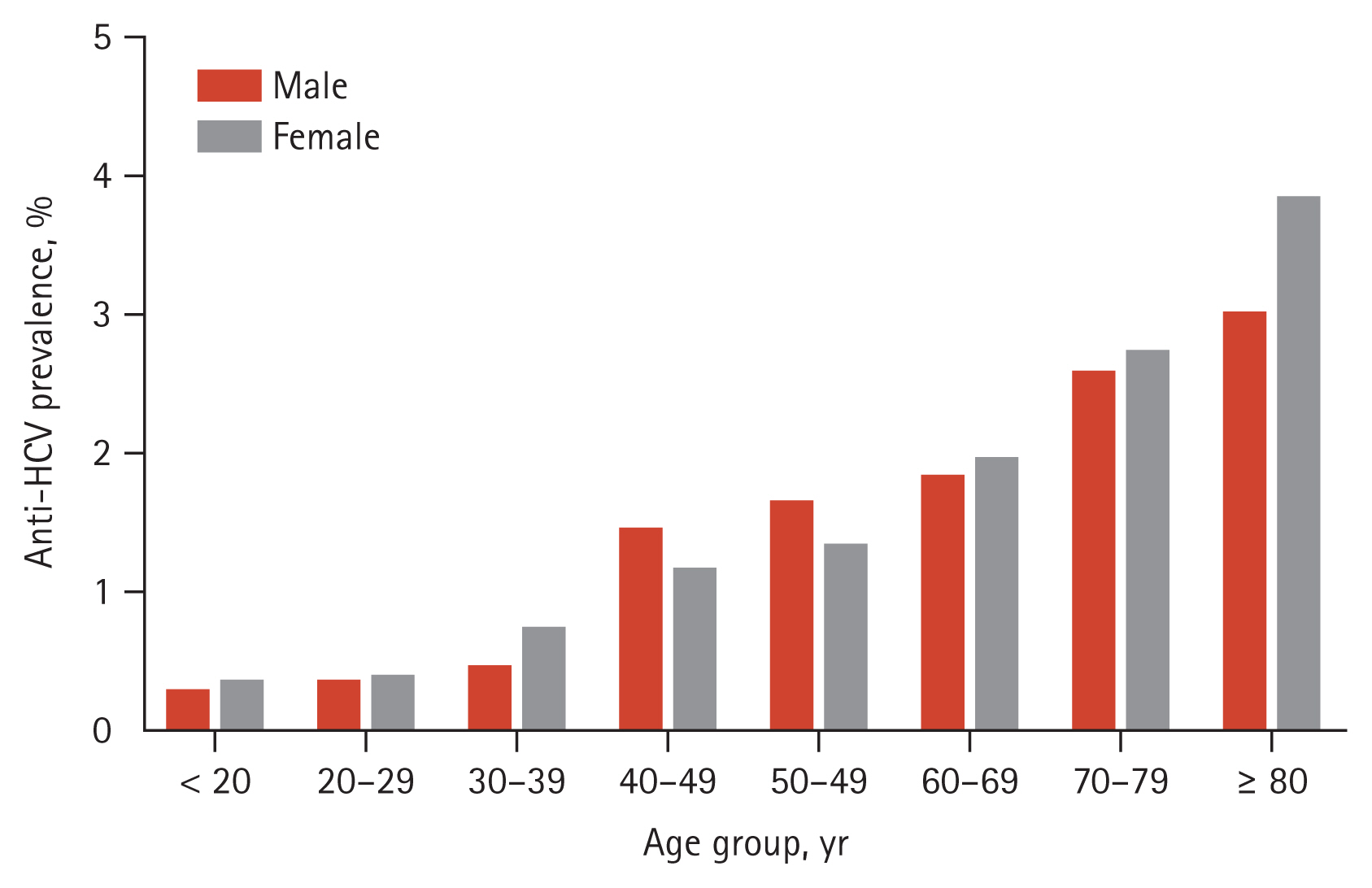
When we applied an anti-HCV S/CO ratio of 5 to define anti-HCV positivity, 610 out of 77,563 patients were determined to be positive, and the crude prevalence of anti-HCV was 0.79%, which was about 45% lower compared to the result using an anti-HCV S/CO ratio of 1. There was no significant difference of anti-HCV prevalence between male (294/39,266; 0.75%) and female patients (316/38,297; 0.83%) (p = 0.238). Age-adjusted anti-HCV positive rates for female patients and male patients were 0.67% and 0.62% respectively. After adjusting for age and sex, the seroprevalence of anti-HCV was 0.65% and was found to increase with age, from 0.03% in individuals under the age of 20 to 1.63% in those aged 80 and above (Fig. 4).
The anti-HCV assay with high sensitivity and specificity has been widely used as a screening test for HCV infection. However, this test has a relatively high FP rate, especially in low-risk populations [10ŌĆō12]. Our study found that 34.7% of patients had FP anti-HCV results, out of all patients in whom TP or FP HCV infection could be ascertained. A previous Korean study showed that about 50% of patients had FP anti-HCV results [23]. In our study, when we applied an anti-HCV S/CO ratio of 5 as the definition for anti-HCV positivity, the prevalence of HCV infection decreased from 1.22% to 0.65%. This indicates that the FP anti-HCV rate was about 45% in our study, which was comparable with the result of a previous Korean study [23]. This indicates that FPs of anti-HCV assays may occur with a high frequency of 45ŌĆō50% in countries like Korea with low HCV prevalence rate of less than 1%. This high rate should be considered at the time of diagnosis as well as in the estimation of HCV infection prevalence. FPs result in the performance of unnecessary additional tests, such as HCV RNA testing and imaging techniques such as ultrasonography. In addition, HCV infection requires continuous monitoring due to the possibility of progression to cirrhosis and HCC. Thus, false positivity may exert various health, economic, and psychological impacts on both patients and providers.
A positive anti-HCV result may indicate a current active infection, a past resolved infection, or an FP reaction. RIBA is commonly used as a supplementary serologic anti-HCV assay to differentiate false positivity from true HCV exposure because of its robust specificity [10]. A positive RIBA result indicates current active or past resolved infection. However, the US Centers for Disease Control and Prevention (CDC) issued an update on the HCV testing approach, including sequential testing of anti-HCV screening-positive samples with the HCV RNA test alone without RIBA due to the unavailability of RIBA and a strategic change in the management of HCV infection from diagnosis to treatment [24]. Despite the CDCŌĆÖs decision to remove RIBA from the diagnostic algorithm for HCV infection, the present study demonstrated that RIBA may still be a useful tool in specific circumstances, such as low prevalence countries like Korea, considering the high FP anti-HCV results and difficulty distinguishing between past resolved and FP HCV infection without RIBA.
In the current study, we were able to differentiate TP from FP HCV infection using RIBA and HCV RNA testing, and we analyzed anti-HCV titers using ROC curves in order to determine an optimal S/CO ratio for the prediction of HCV viremia and TP HCV infection. These determined cutoff values of anti-HCV S/CO ratio for HCV viremia (anti-HCV S/CO ratio of 8) and TP HCV infection (anti-HCV S/CO ratio of 5) could provide clinical significance in the diagnosis of HCV infection, especially in circumstances of unavailability of the RIBA test. In terms of the levels of anti-HCV S/CO ratio for predicting HCV viremia, an anti-HCV S/CO ratio of 8 showed a high diagnostic accuracy, with a sensitivity, specificity, PPV, and NPV of 99.7%, 87.5%, 87.4%, and 99.7%, respectively. This value is comparable with those of previous studies [10,25,26]. Regarding the levels of anti-HCV S/CO ratio for predicting TP HCV infection, we set an anti-HCV S/CO ratio of 5 as an optimal cutoff value, which is applicable to estimate the true prevalence of HCV infection.
Various commercially available anti-HCV assay kits show high agreement in detecting anti-HCV [27]. However, they use different methods and molecular targets for generating and detecting signals and, as a result, may have different levels of diagnostic performance. Some studies reported discrepancies among assays and great variability in cutoff points for predicting TP HCV infection, such as 200 for the Elecsys assay, 19 for the Elecsys II assay, 3ŌĆō5 for the ARCHITECT assay, 7ŌĆō8 for the Vitros assay, 11 for the ADVIA Centaur, and three for the Access assay [27ŌĆō30]. Although anti-HCV assays are highly sensitive and specific, their diagnostic accuracy requires standardization and improvement.
After introduction of highly effective DAA treatment for HCV infection, the HCV management strategy has evolved to focus on the diminution of undiagnosed or untreated patients with HCV infection, which includes scaling up the screening process, reflex HCV RNA testing, and simplification of management. Although these strategies improve HCV infection diagnosis and linkage to care, as well as support earlier treatment initiation, increased screening for HCV infection in a population with a low prevalence rate leads to a higher number of FP results. It is well known that prevalence affects PPV. Therefore, the decline of HCV prevalence after the DAA era further increases FP rates in anti-HCV results. At that time, our study results, an anti-HCV S/CO ratio of 5 for predicting TP HCV infection, may require revision.
The Korean Association for the Study of the Liver recently suggested implementing HCV screening test in the National Health Screening Program (NHSP) as part of an effort toward global elimination of viral hepatitis as a public health problem by 2030. When HCV screening tests are introduced in the NHSP, the high FP rate of anti-HCV assays in Korea should be considered and our study results could provide guidance.
The anti-HCV prevalence of our study (1.22%) was higher than that of previous Korean reports (0.71ŌĆō0.78%), which might be caused by differences in the study population. For example, the participants in our study were usually inpatients, whereas previous Korean studies were conducted on health check-up examinees or the general population. Thus, our study could have included higher-risk populations for HCV infection. Considering the variability of cutoff points of the S/CO ratio for predicting HCV infection according to the types of anti-HCV assays, our results using only the ARCHITECT assay are not applicable to all anti-HCV positive samples. This study also focused on anti-HCV positive results. Therefore, we did not analyze false negativity in anti-HCV assays because we did not evaluate HCV RNA in anti-HCV negative results. This retrospective study could not ascertain TP or FP for HCV infection in all anti-HCV positive patients because not all anti-HCV positive patients were tested for HCV RNA and RIBA. Regardless of these limitations, our study could provide significant clinical information for the diagnosis of TP HCV infection.
In conclusion, because the FP anti-HCV result are unacceptably high in low prevalence areas like Korea, false positivity should be considered and different diagnostic approaches may be required when diagnosing chronic HCV infection. Revision of the anti-HCV S/CO ratio could be useful and applicable to these conditions because it showed high diagnostic accuracy for predicting HCV viremia and TP HCV infection.
1. Of all included patients, 34.7% showed false positive anti-hepatitis C virus (HCV) results.
2. An anti-HCV signal-to-cutoff (S/CO) ratio of 5 was the optimal value to discriminate true positive from false positive HCV infection.
3. Age and sex-adjusted anti-HCV prevalence declined from 1.22% to 0.65%, when applying an anti-HCV S/CO ratio of 5 for anti-HCV positivity.
Notes
CRedit authorship contributions
Jin Gu Kang: conceptualization, data curation, formal analysis, writing - original draft; Myoung Kuk Jang: conceptualization, data curation, formal analysis, project administration, writing - original draft; Jung Hee Kim: conceptualization, formal analysis, methodology, project administration, visualization; Jang Han Jung: conceptualization, formal analysis, methodology, project administration, visualization; Ji Won Park: conceptualization, formal analysis, methodology, project administration, visualization; Sung Eun Kim: conceptualization, formal analysis, methodology, project administration, visualization; Sang Hoon Park: conceptualization, formal analysis, methodology, project administration, visualization; Myung Seok Lee: conceptualization, formal analysis, methodology, project administration, visualization; Ki Tae Suk: conceptualization, formal analysis, methodology, project administration, visualization; Dong Joon Kim: conceptualization, formal analysis, methodology, project administration, visualization; Hyoung Su Kim: conceptualization, data curation, formal analysis, methodology, project administration, visualization, writing - review & editing
Figure┬Ā1
Flow chart of patients enrolled in this study. Anti-HCV, antibodies to hepatitis C virus; RIBA, recombinant immunoblot assay; TP, true-positive; FP, false-positive.
Figure┬Ā3
Receiver operating characteristic (ROC) curves of the antibodies to hepatitis C virus (anti-HCV) signal-to-cutoff (S/CO) ratio for predicting HCV viremia and true positive HCV infection. (A) The area under the ROC (AUROC) of the anti-HCV S/CO ratio for the differentiation of the HCV viremia group from the HCV non-viremia group was 0.970 (95% confidence intervals [CI], 0.959ŌĆō0.982; p < 0.001). (B) The AUROC of the anti-HCV S/CO ratio for the differentiation of true-positive HCV infection from false-positive HCV infection was 0.987 (95% CI, 0.980ŌĆō0.994; p < 0.001).
Figure┬Ā4
Age and sex-specific prevalence of antibodies to hepatitis C virus (anti-HCV) according to an anti-HCV signal-to-cutoff ratio of 5.
Table┬Ā1
Interpretation of results of tests for HCV infection in anti-HCV positive samples
Table┬Ā2
Comparison of the clinical characteristics between the viremia and non-viremia groups
| Total (n = 761) | Viremia (n = 354) | Non-viremia (n = 407) | p value | |
|---|---|---|---|---|
| Age, yr | 59.8 ┬▒ 16.3 | 59.8 ┬▒ 14.5 | 59.7 ┬▒ 17.8 | 0.905 |
| Sex (male) | 367 (48.2) | 172 (48.6) | 195 (47.9) | 0.884 |
| AST, IU/L | 52.5 ┬▒ 93.7 | 68.2 ┬▒ 92.5 | 38.7 ┬▒ 92.8 | < 0.001 |
| ALT, IU/L | 50.7 ┬▒ 109.5 | 68.3 ┬▒ 115.8 | 35.4 ┬▒ 101.3 | < 0.001 |
| Anti-HCV S/CO ratio | 8.37 ┬▒ 5.91 | 13.87 ┬▒ 1.98 | 3.59 ┬▒ 3.58 | < 0.001 |
| RIBAa | < 0.001 | |||
| ŌĆāReactive | 306 (56.9) | 238 (97.1) | 68 (23.2) | |
| ŌĆāIndeterminate | 86 (16.0) | 5 (2.0) | 81 (27.6) | |
| ŌĆāNon-reactive | 146 (27.1) | 2 (0.8) | 144 (49.1) |
Table┬Ā3
Comparison of the clinical characteristics between the TP and FP groups
| Total (n = 649) | TP (n = 424) | FP (n = 225) | p value | |
|---|---|---|---|---|
| Age, yr | 59.6 ┬▒ 15.7 | 60.1 ┬▒ 14.7 | 58.6 ┬▒ 17.3 | 0.254 |
| Sex (male) | 313 (48.2) | 201 (47.4) | 112 (49.8) | 0.621 |
| AST, IU/L | 54.3 ┬▒ 97.2 | 62.1 ┬▒ 86.2 | 39.5 ┬▒ 114.0 | 0.005 |
| ALT, IU/L | 53.6 ┬▒ 115.5 | 61.7 ┬▒ 108.5 | 38.4 ┬▒ 126.6 | 0.015 |
| Anti-HCV S/CO ratio | 9.19 ┬▒ 5.84 | 12.94 ┬▒ 3.26 | 2.12 ┬▒ 1.29 | < 0.001 |
| HCV RNA+a | 354/647 (54.7) | 354/422 (83.9) | 0/225 (0.0) | |
| RIBAb | < 0.001 | |||
| ŌĆāReactive | 308 (57.0) | 308 (97.8) | 0 (0.0) | |
| ŌĆāIndeterminate | 86 (15.9) | 5 (1.6) | 81 (36.0) | |
| ŌĆāNon-reactive | 146 (27.0) | 2 (0.6) | 144 (64.0) |
Table┬Ā4
Diagnostic performance of the anti-HCV S/CO ratio
Table┬Ā5
Clinical characteristics according to the S/CO ratio
| < 5 (n = 509) | 5 and < 8 (n = 63) | Ōēź 8 (n = 546) | |
|---|---|---|---|
| HCV RNA+a | 0/320 (0.0) | 1/37 (2.7) | 353/404 (87.4) |
| RIBAb | |||
| ŌĆāReactive | 20/240 (8.3) | 15/27 (55.6) | 273/280 (97.5) |
| ŌĆāIndeterminate | 74/240 (30.8) | 8/27 (29.6) | 5/280 (1.8) |
| ŌĆāNon-reactive | 146/240 (60.8) | 4/27 (14.8) | 2/280 (0.7) |
| TP HCV infectionc | 20/233 (8.6) | 15/27 (55.6) | 389/389 (100.0) |
REFERENCES
1. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination survey 2001 through 2010. J Hepatol 2014;60:691ŌĆō698.


2. Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol 2022;7:396ŌĆō415.

3. Kim DY, Kim IH, Jeong SH, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int 2013;33:586ŌĆō594.



4. Kim KA, Lee JS. Prevalence, awareness, and treatment of hepatitis C virus infection in South Korea: evidence from the Korea national health and nutrition examination survey. Gut Liver 2020;14:644ŌĆō651.



5. World Health Organization. Global health sector strategy on viral hepatitis 2016ŌĆō2021 [Internet] Geneva (CH): WHO, 2017. [cited 2020 Spe 15]. Available from: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf
.
6. Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2019;4:135ŌĆō184.

7. Pawlotsky JM, Ramers CB, Dillon JF, Feld JJ, Lazarus JV. Simplification of care for chronic hepatitis C virus infection. Semin Liver Dis 2020;40:392ŌĆō402.


8. Ghany MG, Morgan TR, AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020;71:686ŌĆō721.




9. Colin C, Lanoir D, Touzet S, et al. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat 2001;8:87ŌĆō95.


10. Alter MJ, Kuhnert WL, Finelli L, Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep 2003;52:1ŌĆō1315quiz CE1ŌĆōCE4.
11. Hsu HH, Gonzalez M, Foung SK, Feinstone SM, Greenberg HB. Antibodies to hepatitis C virus in low-risk blood donors: implications for counseling positive donors. Gastroenterology 1991;101:1724ŌĆō1727.


12. Conry-Cantilena C, VanRaden M, Gibble J, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med 1996;334:1691ŌĆō1696.


13. Kim YK, Kim BH, Jin ES, et al. Positive predictability and predictive factors of the third generation anti-hepatitis C virus (HCV) ELISA test for HCV infection. Korean J Gastroenterol 2005;45:181ŌĆō188.

14. Moretti M, Pieretti B, Masucci A, Sisti D, Rocchi M, Delprete E. Role of signal-to-cutoff ratios in hepatitis C virus antibody detection. Clin Vaccine Immunol 2012;19:1329ŌĆō1331.



15. Bischoff F, Koch M, Ara├║jo F. Decision on conducting HCV immunoblot and HCV viral load tests dependent upon the result of the screening tests. J Virol Microbiol 2013;2013:332501.

16. Dufour DR, Talastas M, Fernandez MD, Harris B, Strader DB, Seeff LB. Low-positive anti-hepatitis C virus enzyme immunoassay results: an important predictor of low likelihood of hepatitis C infection. Clin Chem 2003;49:479ŌĆō486.



17. Lai KK, Jin M, Yuan S, Larson MF, Dominitz JA, Bankson DD. Improved reflexive testing algorithm for hepatitis C infection using signal-to-cutoff ratios of a hepatitis C virus antibody assay. Clin Chem 2011;57:1050ŌĆō1056.



18. Contreras AM, Ochoa-Jim├®nez RJ, Celis A, et al. High antibody level: an accurate serologic marker of viremia in asymptomatic people with hepatitis C infection. Transfusion 2010;50:1335ŌĆō1343.


19. Contreras AM, Tornero-Romo CM, Toribio JG, et al. Very low hepatitis C antibody levels predict false-positive results and avoid supplemental testing. Transfusion 2008;48:2540ŌĆō2548.


20. Logvinoff C, Major ME, Oldach D, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A 2004;101:10149ŌĆō10154.



21. Nikolaeva LI, Blokhina NP, Tsurikova NN, et al. Virus-specific antibody titres in different phases of hepatitis C virus infection. J Viral Hepat 2002;9:429ŌĆō437.


22. Lu SN, Tung HD, Chen TM, et al. Is it possible to diagnose acute hepatitis C virus (HCV) infection by a rising anti-HCV titre rather than by seroconversion? J Viral Hepat 2004;11:563ŌĆō570.


23. Choi MS, Lee K, Hong YJ, Song EY, Kim DS, Song J. The role of the signal-to-cutoff ratio in automated anti-HCV chemiluminescent immunoassays by referring to the nucleic acid amplification test and the recombinant immunoblot assay. Ann Lab Med 2018;38:466ŌĆō472.




24. Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep 2013;62:362ŌĆō365.


25. Seo YS, Jung ES, Kim JH, et al. Significance of anti-HCV signal-to-cutoff ratio in predicting hepatitis C viremia. Korean J Intern Med 2009;24:302ŌĆō308.



26. Lee CH, Shin HP, Lee JI, et al. Predicting factors of present hepatitis C virus infection among patients positive for the hepatitis C virus antibody. Clin Mol Hepatol 2013;19:376ŌĆō381.



27. Kim S, Kim JH, Yoon S, Park YH, Kim HS. Clinical performance evaluation of four automated chemiluminescence immunoassays for hepatitis C virus antibody detection. J Clin Microbiol 2008;46:3919ŌĆō3923.




28. Cho YK, Kim S, Kim HO, Choi DS, Kim HS, Park Y. Comparative evaluation of Elecsys, Atellica, and Alinity assays for measuring the anti-hepatitis C virus (HCV) antibody. J Clin Virol 2021;141:104910.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



