 |
 |
| Korean J Intern Med > Volume 38(5); 2023 > Article |
|
Abstract
Background/Aims
Methods
Results
Notes
Figure 1
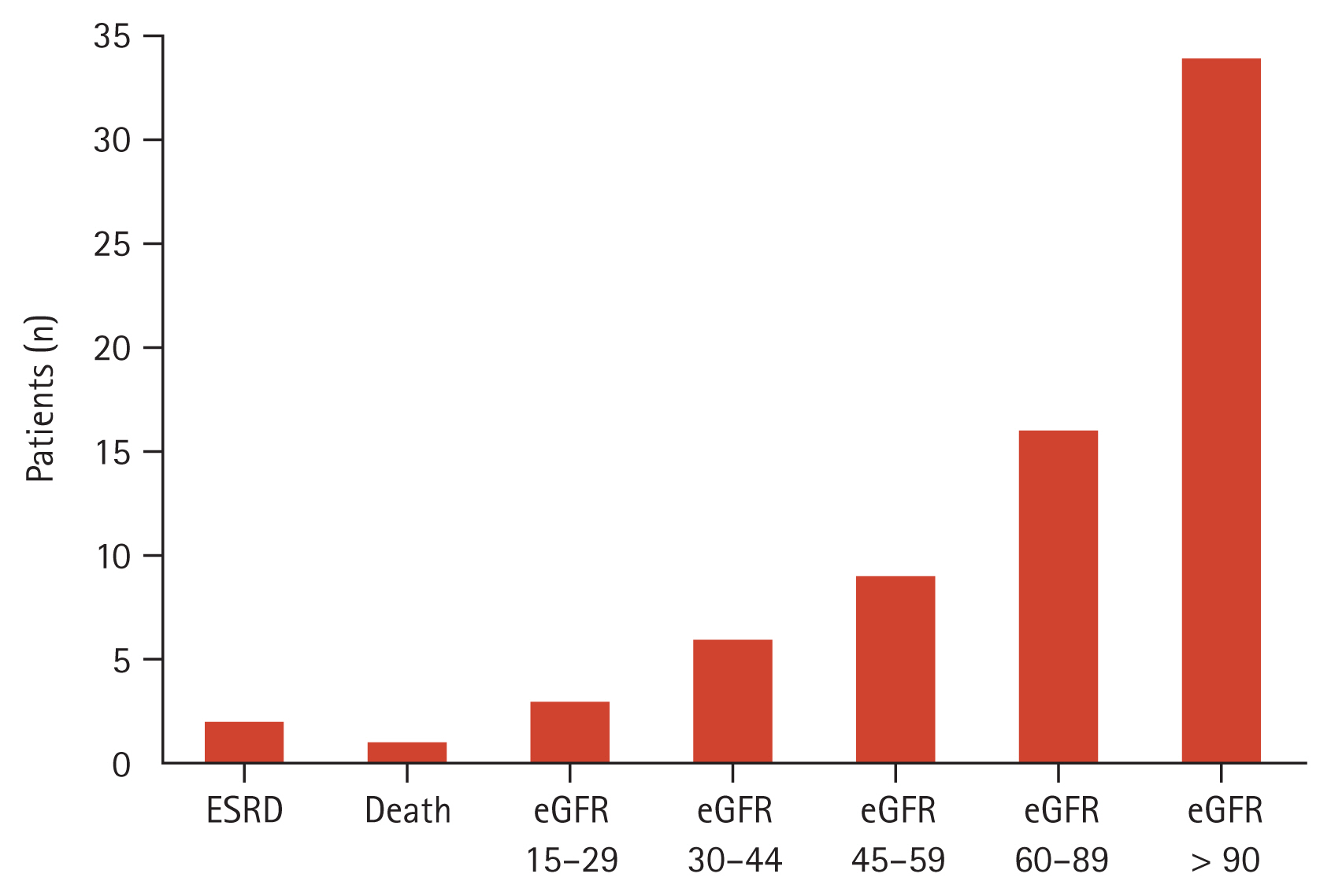
Table 1
| Characteristic | Patient (n = 71) |
|---|---|
| Clinical features | |
| Age, yr | 35 (24–48) |
| Female | 60 (84.5) |
| Hypertension | 34 (47.9) |
| Diabetes mellitus | 7 (9.9) |
| Dyslipidemia | 10 (14.1) |
| SLEDAI | 20 (16–24) |
| Extrarenal SLEDAI | 9 (5–12) |
| Duration from diagnosis of SLE to LN, yr | 2.1 (0.9–3.4) |
| Anti-phospholipid syndrome | 7 (9.9) |
| Laboratory data | |
| ESR, mm/h | 46.92 (26.00–67.00) |
| CRP, mg/dL | 0.40 (0.10–0.57) |
| Creatinine, mg/dL | 0.97 (0.50–1.90) |
| Anti-dsDNA, IU/mL | 58.52 (5.70–35.40) |
| C3, mg/dL | 71.13 (51.20–88.50) |
| C4, mg/dL | 14.50 (7.60–18.90) |
| Albumin, mg/dL | 2.73 (2.30–3.30) |
| Renal profiles | |
| eGFR at LN diagnosis, mL/min/1.73 m2 | 109.69 (82.90–133.80) |
| Urine protein/creatinine, mg/g | 3,716 (1,337–4,614) |
| > 1,000 | 59 (83.1) |
| > 3,000 | 35 (49.3) |
| Biopsy profilesa) | |
| Class I | 4 (5.6) |
| Class II | 17 (23.9) |
| Class V | 48 (67.6) |
| Class II + V | 2 (2.8) |
| Medicationsb) | |
| ACEi/ARB | 46 (64.8) |
| Hydroxychloroquine | 53 (74.6) |
| Corticosteroid | 62 (87.3) |
| Cyclosporine | 18 (25.4) |
| Tacrolimus | 11 (15.5) |
| MMF | 5 (7.0) |
| AZA | 20 (28.2) |
| Medicationsc) | |
| ACEi/ARB | 52 (73.2) |
| Hydroxychloroquine | 64 (90.1) |
| Corticosteroid | 63 (88.7) |
| Tacrolimus | 19 (26.8) |
| MMF | 12 (16.9) |
| AZA | 5 (7.0) |
LN, lupus nephritis; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; ESR, erythrocyte sedimentation rate; CRP, c-reactive protein; anti-dsDNA, anti-double stranded DNA; C3/C4, complement 3/4; eGFR, estimated glomerular filtration rate; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MMF, mycophenolate mofetil; AZA, azathioprine.
Table 2
| Pathologic findings | Patients (n = 71) |
|---|---|
| Activitya) | |
| Hypercellularity | 23 (32.4) |
| Neutrophil/karyorrhexis | 5 (7.0) |
| Hyaline deposits | 9 (12.7) |
| Fibrinoid necrosis | 6 (8.5) |
| Cellular crescents | 3 (4.2) |
| Interstitial inflammation | 32 (45.1) |
| Chronicitya) | |
| Global glomerulosclerosis | 31 (43.7) |
| Fibrous crescents | 6 (8.5) |
| Tubular atrophy | 23 (32.4) |
| Interstitial fibrosis | 16 (22.5) |
| Activity index | 5 (3–7) |
| Chronicity index | 4 (3–5) |
Table 3
HR, hazard ratio; CI, confidence interval; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; anti-dsDNA, anti-double stranded DNA; C3/C4, complement 3/4; eGFR, estimated glomerular filtration rate; LN, lupus nephritis; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CNI, calcineurin inhibitor; MMF, mycophenolate mofetil; AZA, azathioprine.
REFERENCES
- TOOLS
-
METRICS

- Related articles
-
Korean treatment recommendations for patients with axial spondyloarthritis2024 January;39(1)
The underestimated significance of nonproliferative lupus nephritis2023 September;38(5)
Korean treatment recommendations for patients with axial spondyloarthritis2023 September;38(5)
Approach to cytomegalovirus infections in patients with ulcerative colitis2021 May;36(3)
Approach to cytomegalovirus infections in patients with ulcerative colitis2017 May;32(3)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print


