 |
 |
| Korean J Intern Med > Volume 14(2); 1999 > Article |
|
Abstract
Xanthogranulomatous cholecystitis (XGC) is an uncommon, focal or diffuse destructive inflammatory disease of the gallbladder that is assumed to be a variant of conventional chronic cholecystitis. A 36-year-old male was admitted to Chonnam National University Hospital with a 10-day history of right upper quadrant pain with fever. 15 years ago, he was first diagnosed as having hemophilia A, and has been followed up in the department of Hematology. Computed tomogram (CT) revealed a well-marginated, uniform, marked wall thickening of the gallbladder with multiseptate enhancement. Magnetic resonance imaging (MRI) demonstrated diffuse wall thickening of the gallbladder by viewing high signal foci with signal void lesions. After factor VIII replacement, exploration was done. On operation, the gallbladder wall was thickened and the serosa were surrounded by dense fibrous adhesions which were often extensive and attached to the adjacent hepatic parenchyma. There was a small-sized abscess in the gallbladder wall near the cystic duct. Dissection between the gallbladder serosa and hepatic parenchyma was difficult. Cross sections through the wall revealed multiple yellow-colored, nodule-like lesions ranging from 0.5–2cm. There were also multiple black pigmented gallstones ranging from 0.5–1cm. The pathologic findings showed the collection of foamy histiocytes containing abundant lipid in the cytoplasm and admixed lymphoid cells. Histologically, it was confirmed as XGC.
We report a case with XGC mimicking gallbladder cancer in a hemophilia patient.
Xanthogranulomatous cholecystitis (XGC) is an uncommon, focal or diffuse destructive inflammatory disease of the gallbladder that is assumed to be a variant of conventional chronic cholecystitis.
This entity was first described in 1970 by Christen and Ishak as fibroxanthogranulomatous inflammation1). More recently, these terms, together with other labels, such as ceroid granuloma, ceroid-like histiocytic granuloma of the gallbladder2) and biliary granulomatous cholecystitis3), have been abandoned in favor of XGC, a descriptive term first used by McCoy et al4).
XGC is characterized grossly by irregular wall thickening of the gallbladder associated with the formation of yellowish nodules. Histologically, the nodules are predominantly composed of abundant lipid-laden macrophages, inflammatory cells and fibroblasts.
The pathogenesis of XGC is uncertain, but histologic evidence of chronic inflammation is generally seen and gallstones are present in a large majority of cases. It has been suggested that XGC is initiated by a chronic inflammation and obstruction. Occasionally, XGC may closely mimic a gallbladder cancer or lead to complications such as perforation, abscess and fistula.
We report a case with XGC mimicking gallbladder cancer in a hemophilia patient.
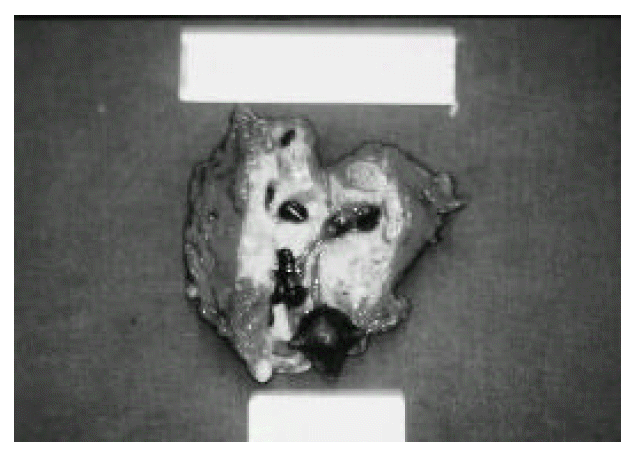
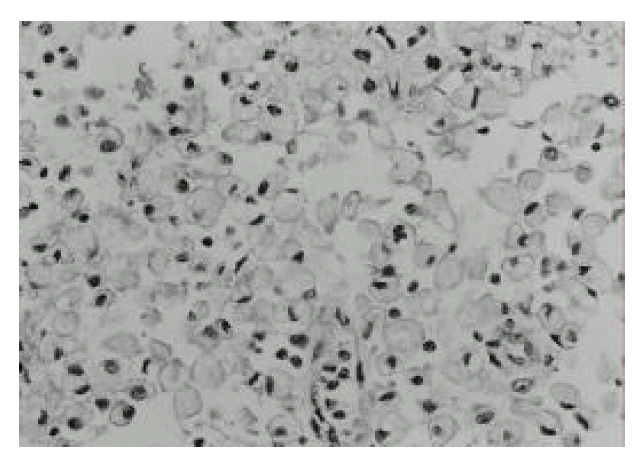
A 36-year-old male was admitted to our hospital with a 10-day history of right upper quadrant pain with fever. 15 years ago, he was first diagnosed as having hemophilia A, and was followed up in the department of Hematology. On admission, the patient was febrile and had tenderness on deep palpation to the right upper quadrant. Rebound tenderness or hepatosplenomegaly was not found. Laboratory studies showed a white blood cell count 15800/mm3, hemoglobin 9.3 g/dL, ESR 141 mm/hr, and CRP 11.3 mg/dL, AST 27 U/L, ALT 16 U/L, ALP 430 U/L. Coagulation profiles were PT 12.8sec (control 11.8sec), aPTT 58.4sec (control from 28 to 40sec) and factor VIII 7.5%(50–150%). Ultrasonographic examination showed an inhomogenous, hypoechoic wall thickening of the gallbladder with internal small stones and no dilatation of intrahepatic bile ducts (Fig. 1). Computed tomogram(CT) revealed a well-marginated, uniform, marked wall thickening of the gallbladder with multiseptate enhancement (Fig. 2). There was no regional lymph node enlargement or focal mass in the liver. Magnetic resonance imaging (MRI) demonstrated diffuse wall thickening of the gallbladder by viewing high signal foci with signal void lesions (Fig. 3). After factor VIII replacement, exploration was done. On operation, the gallbladder wall was thickened and the serosa were surrounded by dense fibrous adhesions which were often extensive and attached to the adjacent hepatic parenchyma. There was a small-sized abscess in the gallbladder wall near the cystic duct. Dissection between the gallbladder serosa and hepatic parenchyma was difficult. On intraoperative cholangiogram through the cystic duct after cholecystectomy, there was no evidence of remaining stone or bile duct dilatation. Cross sections through the wall revealed multiple yellow-colored, nodule-like lesions ranging from 0.5–2 cm. There were also multiple black pigmented gallstones ranging from 0.5–1 cm (Fig. 4). The pathologic findings showed the collection of foamy histiocytes containing abundant lipid in the cytoplasm and admixed lymphoid cells (Fig. 5). Histologically, it was confirmed as XGC. The patient was discharged on postoperative day 10 without complications.
Xanthograulomatous cholecystitis (XGC) is a rare condition of chronic inflammatory disease of the gallbladder. In extensive reports, there is a slightly female predominance, which probably reflects the increased incidence of cholecystitis in women5,6). The lesions occur in a wide age range, but the incidence is higher in the sixth and seventh decades of life6,7). In 78–98% of the patients, gallstones have been demonstrated by ultrasonography8) and obesity and/or diabetes mellitus are common7).
The pathogenesis of XGC is unclear, although the role of lipid and bile is thought to be important. Previous reports have suggested that the important process is the extravasation of bile into the gallbladder wall, either from ruptured Rokitansky-Aschoff sinuses or focal mucosal ulceration2,5). Gallstones are present in most patients. Therefore, obstruction by stones and intraluminal stasis of bile have also been implicated as other important factors9).
The true incidence of XGC is difficult to establish because this disease is apparently a rare condition, although retrospective estimates of the incidence in cholecystectomy specimens range from 0.77) to 9.0 percent10).
Clinical manifestations of XGC are usually those of acute or chronic cholecystitis, but some patients present anorexia, nausea, vomiting, right upper quadrant pain and mass, suggesting gallbladder cancer. XGC may be difficult to distinguish clinically from acute or chronic cholecystitis; radiologically, it is difficult to distinguish from gallbladder cancer. Therefore, before the operation, differential diagnosis of XGC and gallbladder cancer by percutaneous needle biopsy might be helpful in planning the appropriate operative procedure. However, as XGC has the potential for fistula formation, Robert et al6). reported that percutaneous needle biopsy is probably contraindicated, and it may be necessary to confirm the benign nature of the lesion by intraoperative frozen section diagnosis. The most useful indication of percutaneous needle biopsy would, perhaps, be in advanced gallbladder cancer, as unnecessary laparotomy could be avoided. But XGC imitates gallbladder cancer in various ways. Also, there appears to be a positive association between the two lesions. One group reported gallbladder cancer in nearly 10 percent of resected specimens of XGC, higher than the expected incidence of gallbladder cancer in the general population11), and another reported a 10 percent incidence of XGC in reviewed cases of gallbladder cancer12). The reason for this association is not clear. It means that both XGC and gallbladder cancer are complications of gallstone and inflammation of the gallbladder, or it may suggest that tissue disruption by cancer facilitates extravasation of bile into the gallbladder wall13). Despite the possible association between XGC and gallbladder cancer, XGC is not believed to be a premalignant lesion14). Howard et al15), reported that intraoperative cultures of the bile and gallbladder have been positive usually for E. coli, Klebsiella, and/or Enterococcus and, less frequently, for Pseudomonas, Serratia and Staphylococcus aureus. Roberts et al.6) reported that there was a high rate of postoperative infective complication, with one subphrenic abscess and three wound infections (one fatal), two of the patients with fistula. They are likely to be complicated by the presence of dense fibrous adhesions, abscess and adherence of the gallbladder to adjacent structures.
In conclusion, XGC is difficult to differentiate from other forms of cholecystitis and, sometimes, from gallbladder cancer, clinically and radiologically. XGC may be a high risk of postoperative wound infection and other septic complications because of frequent adhesion and abscess formation.
Fig. 1.
Ultrasonographic examination showed an inhomogenous, hypoechoic wall thickening of the gallbladder with internal small stones, and no dilatation of intrahepatic bile ducts.
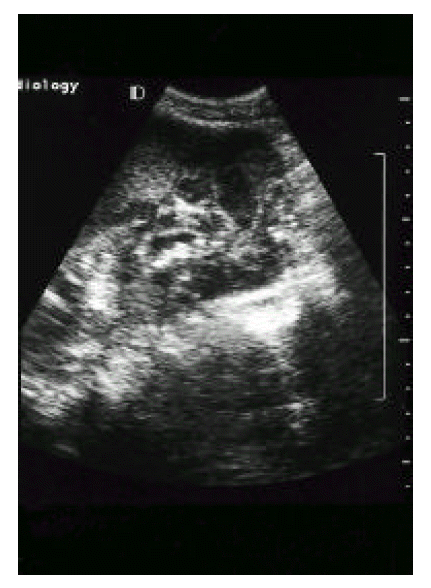
Fig. 2.
Computed tomogram (CT) revealed a well-marginated, uniform, marked wall thickening of the gallbladder with multiseptate enhancement. There was no regional lymph node enlargement or focal mass in the liver.
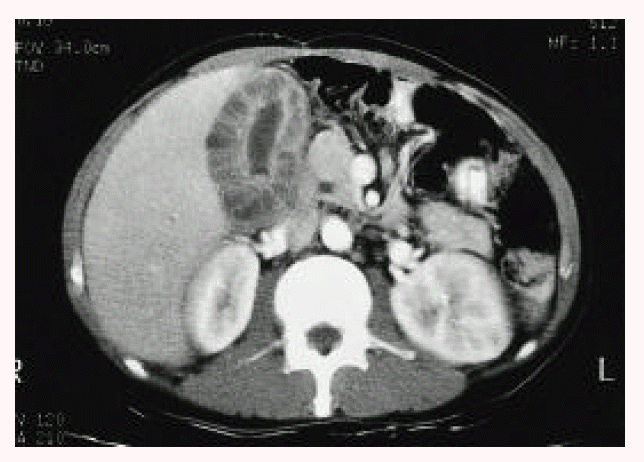
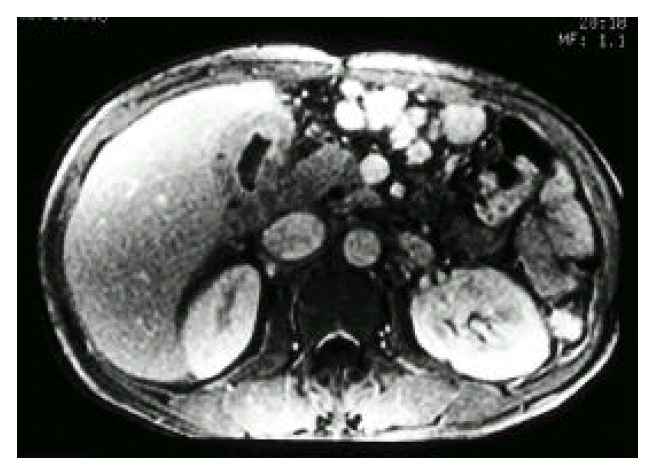
Fig. 3.
Magnetic resonance imaging (MRI) demonstrated diffuse wall thickening of the gallbladder by viewing high signal foci with signal void lesions.
REFERENCES
1. Christensen AH, Ishak KG. Benign tumors and pseudotumors of the gallbladder: report of 180 cases. Arch Pathol 1970;90:423–432.

2. Takahashi K, Oka K, Hakozaki H, Kojima M. Ceroidlike histiocytic granulomas of gallbladder. A previously undescribed lesion. Acta Pathol Jpn 1976;26:25–46.

3. Mehrotra ML, Bhatnagai BNS. Biliary granulomatous cholecystitis. J Indian Med Assoc 1982;79:144–145.

4. McCoy JJ, Vila R, Petrossian G, McCall RA, Reddy KS. Xanthogranulomatous cholecystitis: report of two cases. J S C Med Assoc 1976;72:78–79.

6. Roberts KM, Parsons MA. Xanthogranulomatous cholecystitis: clinicopathological study of 13 cases. J Clin Pathol 1987;40:412–417.



7. Reyes CV, Jablokow VR, Reid R. Xanthogranulomatous cholecystitis: report of seven cases. Am Surg 1981;47:322–325.

8. Franco V, Aragona F, Genova G, Florena AM, Stella M, Campesi G. Xanthogranulomatous cholecystitis: histopathological study and classification. Pathol Res Pract 1990;186:390.

9. Fligial S, Lewin KJ. Xanthogranulomatous cholecystitis. Arch Pathoi Lab Med 1982;106:302–304.
10. Hanada K, Nakata H, Nakayama T, Tsukamoto Y, Terashima H, Kuroda Y, Okuma R. Radiologic findings in xanthogranulomatous cholecystitis. AJR 1987;148:727–730.


13. Bebow EW. Xanthogranulomatous cholecystitis associated with carcinoma of gallbladder. Postgrad Med J 1989;65:528–531.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



