Efficacy and safety of 1 L polyethylene glycol plus ascorbic acid for bowel preparation in elderly: comparison with oral sulfate solution
Article information
Abstract
Background/Aims
Recently, 1 L of polyethylene glycol (PEG) plus ascorbic acid (Asc) has been introduced in Korea as a colonoscopy preparation agent. Data on its efficacy and safety in older adults have been limited. We aimed to evaluate the safety and efficacy of 1 L PEG/Asc in older adults by comparing it with oral sulfate solution (OSS).
Methods
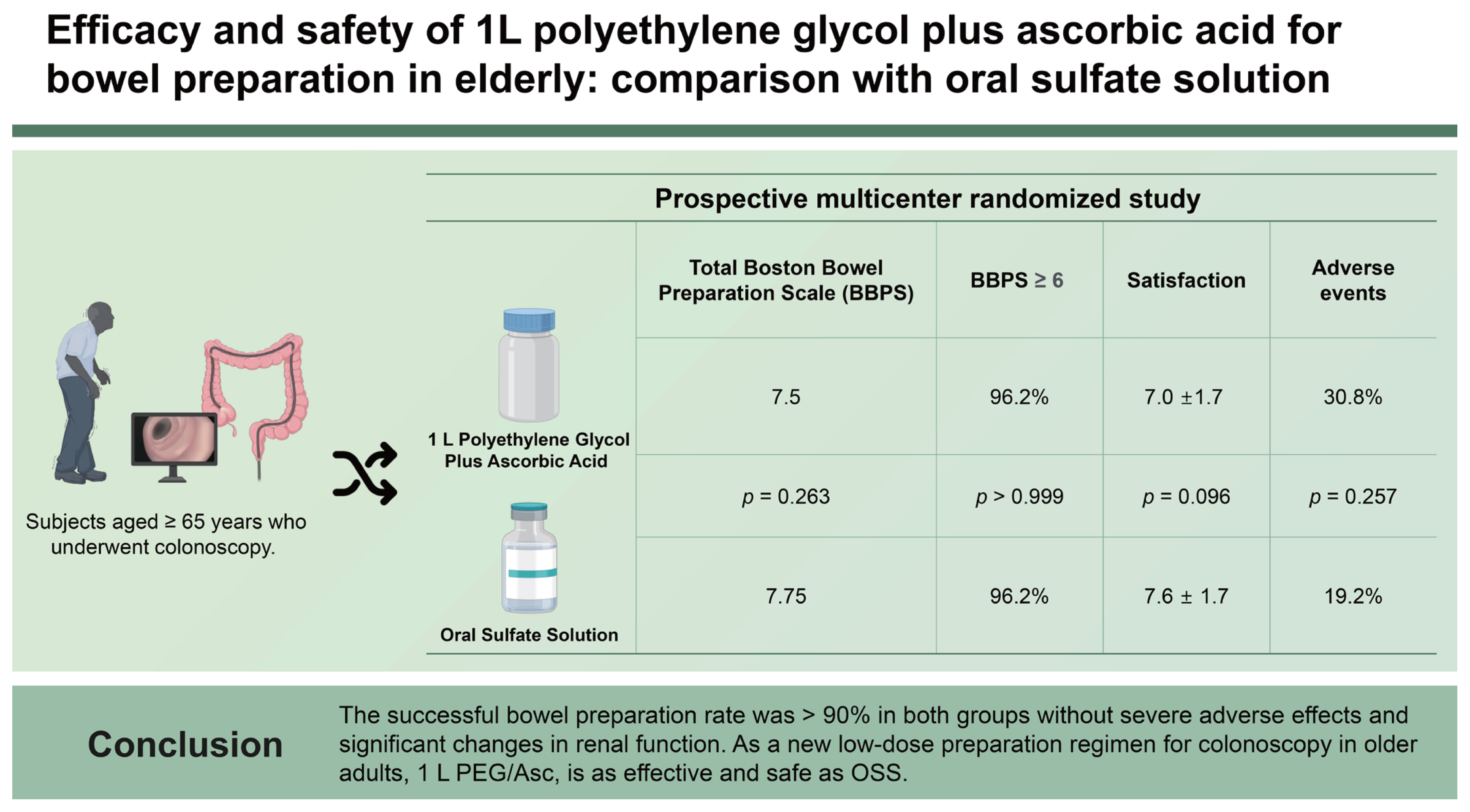
A prospective multicenter randomized study was conducted with subjects aged ≥ 65 years who underwent colonoscopy. The participants were randomized to receive 1 L PEG/Asc or OSS. The primary endpoint was successful bowel preparation, defined as total Boston Bowel Preparation Scale ≥ 6, and ≥ 2 at each segment. Patient satisfaction, adverse events, and renal function changes were compared between the groups.
Results
Among the 106 patients, 104 were finally included in the analysis. Overall, successful bowel preparation was achieved in 96.2% of both 1 L PEG/Asc and OSS groups. The satisfaction scores for taste, total amount ingested, overall feeling, and willingness to repeat the same regimen were not significantly different between the groups. Adverse events of moderate or higher severity occurred in 16 and 10 cases in the 1 L PEG/Asc and OSS group, respectively. There were no significant changes in electrolyte levels or renal function from baseline.
Conclusions
The successful bowel preparation rate was > 90% in both groups without severe adverse effects and significant changes in renal function. As a new low-dose preparation regimen for colonoscopy in older adults, 1 L PEG/Asc, is as effective and safe as OSS.
INTRODUCTION
Colonoscopy with removal of premalignant lesions has been considered the most effective colorectal cancer (CRC) screening tool because most CRCs arise from adenomatous polyps [1,2]. Considering that the incidence of colorectal adenoma and cancer increases with age, a large proportion of colonoscopies are performed on older adults [3]. Although adequate bowel preparation is crucial for a full inspection of the colonic mucosa and removal of precancerous lesions, older adults are at higher risk of poor bowel preparation due to slower colonic transit and higher prevalence of obstipation [4]. In addition, they are less tolerant to large-volume preparation agents than younger patients [5]. Various low-volume preparation agents, such as polyethylene glycol (PEG) and non-PEG-based agents, have been introduced to enhance tolerability and adherence. Most of the agents have proven to be non-inferior in efficacy and safety compared with 4 L PEG [6–13]. However, most studies regarding novel low-volume preparation agents have excluded older adults. Therefore, the 4 L PEG-based split dose preparation is still accepted as safe and effective in these age groups despite reduced adherence due to the large volume [14]. Considering that older adults are at higher risk of colorectal neoplasms and have low tolerability to ingest large volumes of preparation agents, it could be very helpful if low-volume preparation is effective and safe for them.
Recently, 1 L PEG plus ascorbic acid (1 L PEG/Asc) was introduced in Korea. Although its efficacy and safety have been approved by several studies, data on older adults have been limited. Therefore, we aimed to evaluate the safety and efficacy of 1 L PEG/Asc in older adults by comparing it with another non-PEG-based low-volume preparation, oral sulfate solution (OSS).
METHODS
Study population and design
This prospective, randomized, non-inferiority, investigator-blinded, multicenter study was conducted at five academic hospitals in Korea from September 2019 to August 2020. Eligible patients were consecutive older adult outpatients aged between 65 and 84 years who had undergone screening or surveillance colonoscopy for colon polyp and CRC. The exclusion criteria were as follows: (1) previous history of colectomy or gastrectomy; (2) inflammatory bowel disease; (3) severe constipation; (4) intestinal obstruction; (5) severe congestive heart failure (New York Heart Association [NYHA] class III or IV); (6) acute myocardial infarction in the preceding six months; (7) severe renal insufficiency (creatinine clearance rate < 30 mL/min); (8) liver cirrhosis; and (9) American Society of Anesthesiologists physical status index ≥ III.
Participants who provided informed consent in each hospital were randomly assigned to receive computer-generated random numbers into 1 L PEG/Asc or OSS groups at a 1:1 ratio. The investigators did not know which regimen was assigned to the participants until study completion.
Bowel preparation protocol
Patients were 1:1 randomized to receive the bowel cleansing regimens: (1) 1 L PEG/Asc (CleanViewAL; Taejoon Pharm, Seoul, Korea; composition: PEG 3350, 160 g; sodium chloride, 2.7 g; potassium chloride, 1.0 g; anhydrous sodium sulfate, 18 g; Acs, 40.6 g; and sodium ascorbate, 9.4 g) and (2) OSS (Suprep; Taejoon Pharm; composition: sodium sulfate, 35 g; potassium sulfate, 6.26 g; magnesium sulfate, 3.2 g).
All enrolled participants were instructed by a nurse to consume a low-fiber diet three days before colonoscopy, and a rice porridge at 5 p.m. the day before the examination. The preparations were dispensed by a nurse who carefully explained how they should be taken, emphasizing the importance of complete intake of the solution to ensure a safe and effective procedure.
All preparations were performed using a split dose. The first and second dose were administered between 6:00 and 8:00 p.m. on the day before the colonoscopy and 6:00 and 8:00 a.m. on the day of the colonoscopy, respectively. Patients in the 1 L PEG/Asc group drank 500 mL of Clean-ViewAL solution, with an additional 500 mL of plain water in the evening before the colonoscopy. Patients in the OSS group drank 473 mL Suprep solution, which was a mixture of a bottle of Suprep and plain water, followed by the same amount of plain water in the evening before the colonoscopy. The same procedures were repeated with the same agents on the morning of the colonoscopy in both groups. The preparations were completed at least 2 hours prior to the examination, and colonoscopies were performed within 6 hours of the last dose prepared. All patients underwent colonoscopy in the morning between 9:00 a.m. and noon.
Assessment of outcomes
Efficacy of bowel preparation
The primary endpoint was the successful bowel preparation rate using the Boston Bowel Preparation Scale (BBPS), which was defined as a score ≥ 2 for each segment and a total score ≥ 6. Using BBPS, the degree of bowel cleansing was rated on a scoring scale of 0 to 3 for each anatomical segment of the colon (right, transverse, and left segment): 0 (unprepared colon segment with mucosa not seen due to solid stool that could not be cleared); 1 (portion of mucosa of the colon segment seen, but other areas of the colon segment not well seen due to staining, residual stool, and/or opaque liquid); 2 (minor amount of residual staining, small fragments of stool and/or opaque liquid, but mucosa of colon segment seen well); and 3 (entire mucosa of colon segment seen well, with no residual staining, small fragments of stool, or opaque liquid) [15,16]. The other secondary endpoints were perfect bowel preparation rate defined as a score ≥ 3 for all segments and total score = 9, cecal intubation rate, average withdrawal time, and adenoma detection rate (ADR).
Bowel cleansing and other outcomes were assessed by the endoscopists performing the procedure, each of whom had at least 10 or more years of experience performing colonoscopies, and was unaware of the preparation method. To reduce inter-observer variability, all participating endoscopists were trained with captured colonoscopy sample images before study initiation. The preparation score was assessed by endoscopists as soon as the colonoscopy was completed, and reference images were provided in each case report form for standardized assessment.
Tolerability and safety
On the day of colonoscopy, all participants completed a questionnaire related to tolerability by the study nurse before the procedure. Satisfaction and tolerability in terms of taste, amount, and overall feeling were assessed using a 10-level visual analog scale (VAS). We categorized the scores into five grades: very bad, bad, moderate, good, and very good. If the score was higher than 6, we considered it to be good. Complete ingestion rate and willingness to repeat the same regimen were also assessed.
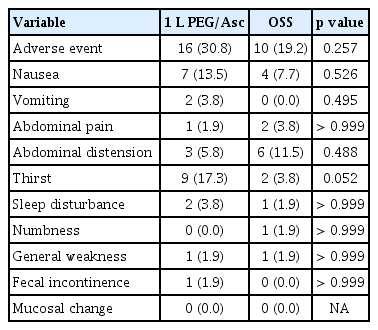
Any adverse events related to bowel preparation, such as nausea, vomiting, abdominal pain, abdominal distension, thirst, sleep disturbance, numbness, general weakness, fecal incontinence, convulsion, change of consciousness, and anuria, were also evaluated by the study nurse before colonoscopy. One adverse event, thirst, was assessed based on the patient’s feeling of dry mouth. These symptoms were rated on a 5-point scale (none, mild, moderate, severe, or very severe). In addition, mucosal changes defined as the appearance of aphthous ulcers, ulcers, and erythema in the colonic mucosa due to preparation were compared.
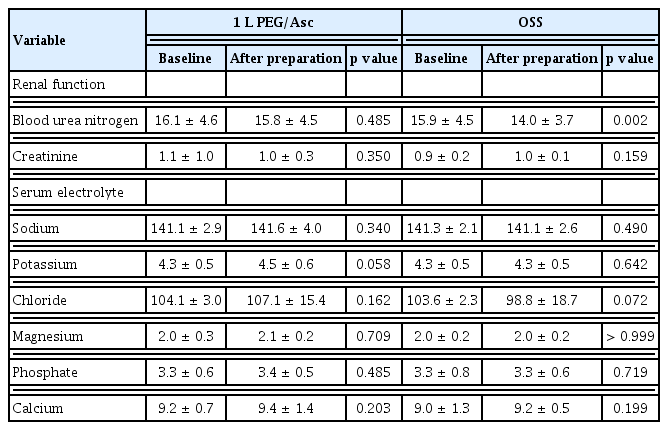
Changes in renal function or serum electrolyte levels before and after preparation were compared between the groups. Blood tests to assess renal function and electrolyte concentrations were performed on the day of colonoscopy immediately after preparation, and compared with the results obtained at the screening visit for the baseline study.
Statistical analysis
We assumed that successful bowel preparation would be achieved in 90% of the patients in the OSS group based on a previous study [8]. The sample size required for 80% power to detect a 15% difference in successful bowel preparation rate with a two-sided significance level of 0.05 was estimated as 48 in each group. Considering a dropout rate of 10%, a total of 106 patients (53 in each group) were needed to prove the non-inferiority of 1 L PEG/Asc.
Categorical variables are expressed as numbers and rates, while continuous variables are described as mean ± standard deviation or median (range). Chi-square or Fisher’s exact tests were used to compare categorical variables, while Student’s t-test was used to compare continuous variables. Statistical significance was identified at a two-sided p < 0.05. Statistical analyses were performed using IBM SPSS Statistics ver. 20 for Windows (IBM Corp., Armonk, NY, USA).
Ethics statement
The study protocol was registered at cris.nih.go.kr (KCT0004224), and approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (YUMC 2019-07-016) and all participating hospitals. The data underlying this article will be shared upon reasonable request by the corresponding author.
RESULTS
Patients’ baseline characteristics
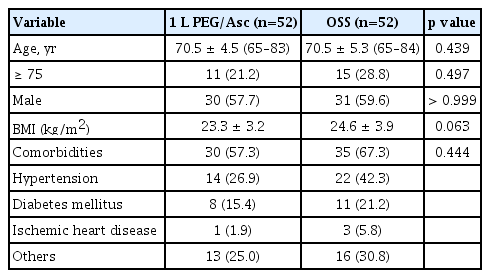
A total of 106 patients were randomized to receive either 1 L of PEG/Asc (n = 53) or OSS (n = 53). Of these, one patient in each group withdrew consent and was excluded from the analyses. Accordingly, 52 patients in both groups were included. The baseline characteristics were well-balanced between the groups, with no significant differences. The median age of the patients in 1 L-PEG/Asc and OSS groups was 70.5 ± 4.5 years (range, 65–83 yr) and 70.5 ± 5.3 years (range, 65–84 yr), respectively, and the male-to-female ratio did not significantly differ between groups. Twenty-six patients (25.0%) were aged > 75 years, 11 (21.2%) in the 1 L PEG/Asc group, and 15 (28.8%) in the OSS group. The proportions of patients with comorbidities were 57.3% and 67.3%, respectively (Table 1).
Efficacy of bowel preparation
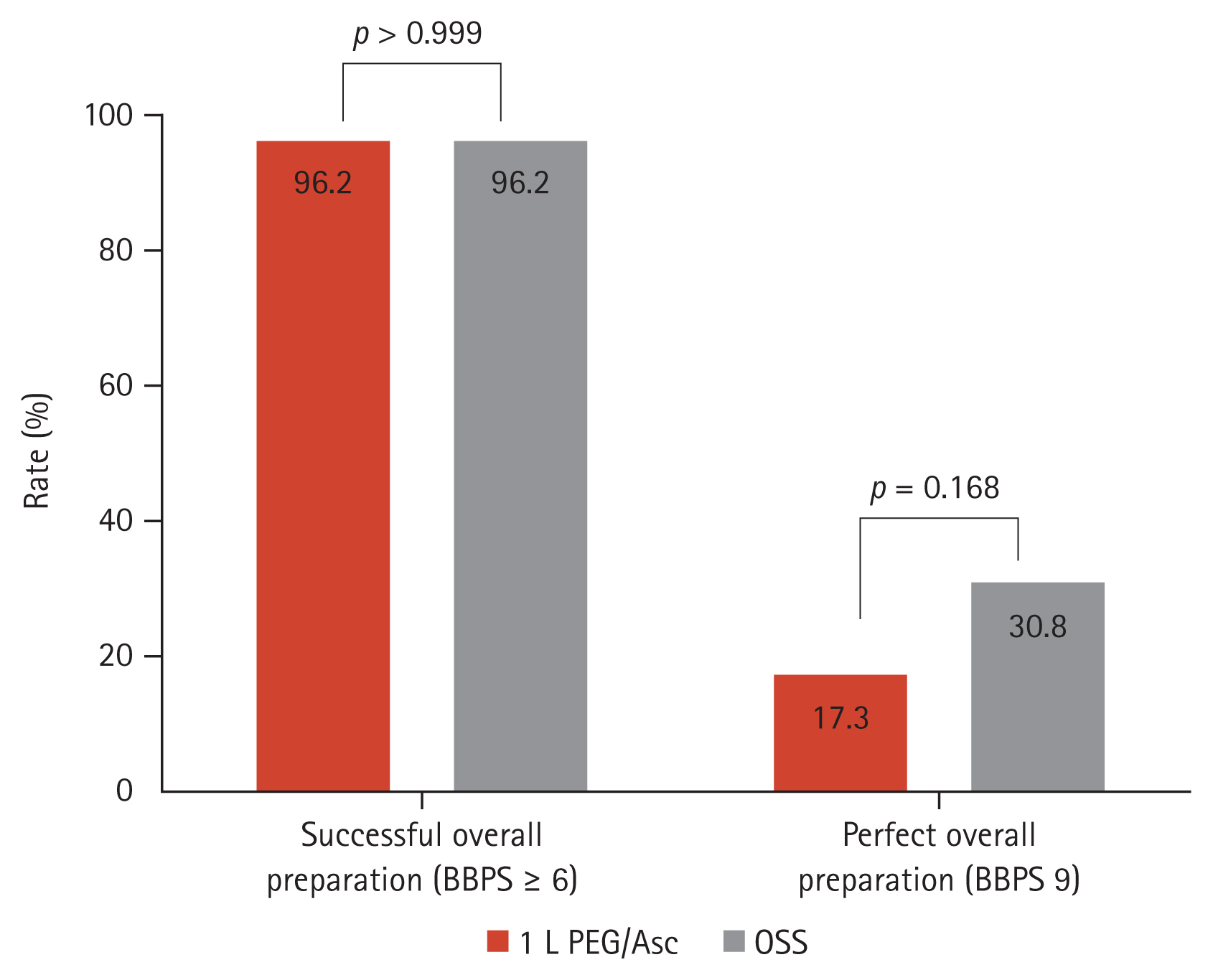
Cecal intubation was achieved in 100% of the 1 L PEG/Asc group versus 98.1% (51/52) of the OSS group (p > 0.999). The withdrawal time did not differ between groups (775.9 ± 536.9 s vs. 828.5 ± 506.7 s, p = 0.609). The mean overall BBPS (7.50 ± 1.1 vs. 7.75 ± 1.1, p = 0.263), and the mean score at each segment (right, transverse, and left colon) was not statistically different either (Fig. 1). The overall successful bowel preparation rate was 96.2% (50/52) in both the 1 L PEG/Asc and OSS groups. The successful bowel preparation rate at each segment was not significantly different between groups. The overall perfect bowel preparation (BPPS 9) was achieved in 17.3% (9/52) of the 1 L PEG/Asc group versus 30.8% (16/52) of the OSS group, with no significant difference (p = 0.168) (Fig. 2). The perfect preparation rate for each segment was not significantly different between the groups. The overall ADR was non-inferior in the 1 L PEG/Asc compared to the OSS group (55.8% vs. 61.5%, p = 0.691).

BBPS at each segment. BBPS, Boston Bowel Preparation Scale; OSS, oral sulfate solution; PEG/Asc, polyethylene glycol/ascorbic acid.
Tolerability and safety
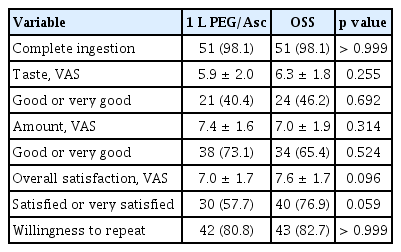
Complete purgative ingestion was reported in 98.1% (51/52) of patients in both the 1 L PEG/Asc and OSS groups. The reasons for failure were the taste of the agents and nausea in the 1 L PEG/Asc and OSS group, respectively. Satisfaction score regarding taste (5.9 ± 2.0 vs. 6.3 ± 1.8, p = 0.255), total amount ingested (7.4 ± 1.6 vs. 7.0 ± 1.9, p = 0.314), and overall feeling (7.0 ±1.7 vs. 7.6 ± 1.7, p = 0.096) were not significantly different between groups. The proportion of patients with good (VAS 7–8) or very good (VAS 9–10) taste, total amount ingested, and overall satisfaction were not significantly different. Both groups of patients showed a willingness to repeat the same purgatives at the next examination in > 80% of cases (p > 0.999) (Table 2).
Adverse events of moderate or higher severity occurred in 16 cases of 1 L PEG/Asc and 10 cases in the OSS group. In 1 L PEG/Asc, thirst was the most common (nine cases), followed by nausea (seven cases). In the OSS group, abdominal distension (six cases) was the most common, followed by nausea (four cases). The frequency of adverse events was not significantly different between groups (Table 3). No serious adverse events or deaths were reported. During colonoscopy, there were no mucosal changes in either group, such as erosion or erythema.
Electrolyte and renal function changes associated with bowel preparation did not occur in the 1 L PEG/Asc group. Although blood urea nitrogen in the OSS group showed a significant numerical change (p = 0.002), it was not considered clinically meaningful because it was below the normal upper range (Table 4). In addition, no clinically significant events were associated with renal function.
Subgroup analysis by age group
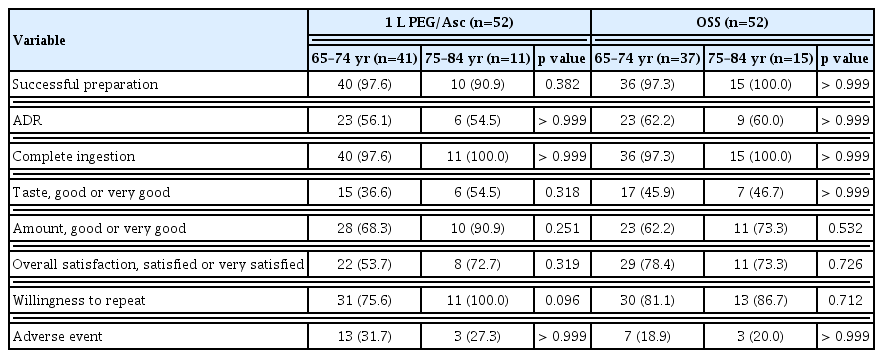
We also performed a subgroup analysis according to age (65–74 yr, n = 78; 75–84 yr, n = 26). There were no significant differences in the successful bowel preparation rate (96.2% vs. 96.2%, p > 0.999) and overall ADR (59.0% vs. 57.7%, p = 0.543) between the subgroups. Additionally, overall satisfaction (65.4% vs. 73.1%, p = 0.630), willingness to repeat (78.2% vs. 92.3%, p = 0.146), and adverse events of moderate or higher severity (25.6% vs. 23.1%, p > 0.999) were not significantly different between the groups.
In addition, we compared age with a cutoff value of 75 years in both the 1 L PEG/Asc and OSS groups. There were no significant differences in efficacy (successful bowel preparation rate and overall ADR), tolerability, and safety (complete ingestion rate, taste, amount, overall satisfaction, willingness to repeat, and adverse events) between subjects < and ≥ 75 in both the 1 L PEG/Asc and OSS groups (Table 5).
DISCUSSION
As the incidence of CRC increases with age, colonoscopy in older adults has increased parallel to life expectancy [17,18]. In general, osmotically balanced 4 L PEG solutions are thought to be the safest, and are preferred in older adults [14]. However, older adults often have difficulties taking large amounts of preparation agents, so they fail to achieve adequate bowel preparation [19]. Fortunately, in addition to 4 L PEG, which is currently considered a conventional standard agent, various low-volume bowel preparation agents such as 2 L PEG/Asc, 1 L PEG/Asc, and non-PEG-based agents such as OSS have been released [19,20]. Several studies have been conducted to compare the efficacy, tolerability, and safety of these agents [8,12,20–25]. One of these studies, comparing OSS with 4 L PEG and enrolling patients > 65 years, showed that OSS as low-volume agents was not inferior (in terms of efficacy, safety, and tolerability) to the 4 L PEG [8]. However, this study has a limitation because older adults > 75 years and those with comorbidities were excluded. Another study comparing 1 L PEG/Asc with OSS in patients of all ages showed no significant differences between the 1 L PEG/Asc and OSS groups. However, the age of the patients in this study ranged from 20 to 71 years, and most of them were < 65 years [26]. Therefore, we evaluated the efficacy, safety, and tolerability of low-volume preparation agents by comparing both 1 L PEG/Asc and OSS in older adults, including 65 to 84 years and with comorbidities. Our results demonstrated that 1 L PEG/Asc was similarly tolerable, safe, and effective compared to OSS for bowel preparation in older adults.
Our results showed no significant difference in the successful preparation rate between low-volume preparation agents. The preparation scores for each segment were similar in both groups. For proper colonoscopy, the Quality Committee of the European Society of Gastrointestinal Endoscopy recommends a minimum standard of ≥ 90% for adequate bowel preparation [27]. In our study, the overall successful preparation rate by BPPS (≥ 6) was 96.2% in both the 1 L PEG/Asc and OSS groups.
ADR is considered the primary indicator of mucosal inspection quality and the single most important quality measure in colonoscopy [28]. In our study, the ADR of the 1 L PEG/Asc and OSS groups were 55.8% and 61.5%, respectively, exceeding the target of 25% recommended by the American Society for Gastrointestinal Endoscopy for screening colonoscopies [29–32]. ADR varies depending on the population, purpose of endoscopy, and techniques such as cecal intubation rate, withdrawal time, and bowel preparation level. Thus, every study showed a variable degree of ADR. On average, ADR was higher in studies targeting the elderly than for all age groups. For example, some studies targeting all ages showed ADR from 18.7 to 36.6, and some studies targeting elderly individuals showed ADR from 47.1 to 69.8 [20,22,33–35]. Considering the above results, ADR was somewhat higher in our study rather than other studies because we included elderly patients and the incidence of colorectal adenoma increases with age. In addition, since our study included surveillance colonoscopy as well as screening, ADR might be higher than that in other studies.
The satisfaction with taste, total amount ingested, and overall feeling showed no significant differences. Bowel preparation-related adverse events were not significantly different between groups. Compared with previous studies on 1 L PEG/Asc or OSS, the results were similar [8,35]. Because both 1 L PEG/Asc and OSS are low-volume agents, there might be no significant differences in tolerability.
We also compared the efficacy, tolerability, and safety between the subgroups by age and found no significant differences. This shows that low-volume agents are also efficient, tolerable, and safe, even in older adults > 75 years.
The reason why we chose OSS as the control group was that it already showed similar efficacy and safety with superior tolerability compared to 4 L PEG in a previous study in older adults [8]. So, we assumed that it could be a good alternative to 4 L PEG as a preparation agent in older adults if 1 L PEG/Asc was not inferior to OSS.
Our study had several limitations. First, since uncontrolled comorbidities such as severe heart failure, renal failure, and acute myocardial infarction were excluded due to a lack of safety assurance, our results regarding the efficacy and safety of 1 L PEG/Asc were not applicable to older adults with these comorbidities. Additional research should be conducted on older adults with severe comorbidities and structural changes. Second, when the medical staff provided education on diet control and medication regimen before the test, there might have been differences in the patient’s acquisition level or reflection of the educational content because our study population was > 65 years old. To reduce this difference, we used the same diet leaflet in all participating hospitals. Third, because a considerable number of subjects had at least one type of comorbidity and took medication, they could be one of confounding factors. Fourth, although the minimum number of samples for each group to obtain appropriate results was satisfied, the number of patients older than 75 years was too small. Therefore, we need more study with larger number of patients in this age group. Lastly, we could not exclude the possibility of inter-observer variability in the assessment of the preparation efficacy. To reduce inter-observer variability, all participating endoscopists were trained with captured colonoscopy sample images before study initiation. We reported the preparation score as soon as the colonoscopy was finished in a case report form, at which a standard image was presented for the standardized assessment.
Despite these limitations, our study has several strengths in that we targeted elderly patients up to the age of 84 years compared with a previous study that included patients aged < 75 years [8,26]. And this is the first study comparing 1 L PEG/Asc and OSS in only elderly patients. In addition, it is significant that the elderly group aged 65 to 84 was classified into subgroups (< and > 75 yr), and the efficacy and safety were compared again. By using OSS, whose efficacy and safety have been confirmed in previous studies, as a comparison group, our study confirmed the results of a previous study. While most of the previous studies usually compared high volume agents and novel low-volume agents, our study is meaningful in that we compared novel low-volume agents against each other in elderly subjects.
In conclusion, both 1 L PEG/Asc and OSS showed acceptable preparation efficacy and safety with high tolerability in patients older than 65 years. Based on these results, 1 L PEG/Asc could be considered as an alternative to 4 L PEG in the older adults.
KEY MESSAGE
1. This is a prospective randomized controlled study to evaluate the efficacy, safety and tolerability of 1 L of PEG/ACs in older adults by comparing it with OSS.
2. Both 1 L PEG/Asc and OSS showed acceptable preparation efficacy and safety with high tolerability in patients older than 65 years. Based on these results, 1 L PEG/Asc could be considered as an alternative to 4 L PEG in the older adults.
Notes
Conflicts of interest
The authors disclose no conflicts.
CRedit authorship contributions
Ki Young Lim: data curation, formal analysis, writing - original draft; Kyeong Ok Kim: conceptualization, formal analysis, writing - review & editing, funding acquisition; Eun Young Kim: data curation, methodology, project administration; Yoo Jin Lee: data curation, methodology, visualization; Byung Ik Jang: data curation, formal analysis, methodology; Sung Kook Kim: data curation, formal analysis, visualization; Chang Heon Yang: data curation, formal analysis, methodology
Funding
This study was supported by Taejoon Pharm Inc., Seoul, Korea, as an investigator-initiated study.