 |
 |
| Korean J Intern Med > Volume 7(2); 1992 > Article |
|
Abstract
Reports on the histologic findings of chronic active hepatitis C (CAH-C) have been rare, and the characteristic histologic findings of CAH-C have been not yet determined. To compare the differences in the histologic findings between CAH-C and chronic active hepatitis B (CAH-B) group, we analyzed the histologic findings of 19 patients with CAH-C, who had positive tests for HCV-antibody by EIA, and 19 patients with CAH-B who had negative tests for HCV-antibody but positive tests for HBsAg by RIA. Histologic features were analyzed between the CAH-C and CAH-B groups using a scoring system which is modified from Knodell’s histologic activity index-looking at portal inflammation, periportal necroinflammation, portal fibrosis, focal necrosis, regeneration, polyploid nuclear change, sinusoidal lymphocytic reaction and fatty change.
Portal inflammatory cell infiltrations with prominent lymphocytes and follicular arrangement were more frequent in the CAH-C group (10 of 19 cases) than in the CAH-B group (5 of 19 cases). Severe sinusoidal lymphocytic reactions were also more prominent in the CAH-C group (11 of 19 cases) than in the CAH-B group (6 of 19 cases). However, periportal necroinflammation, portal fibrosis, focal hepatic necrosis, regeneration and polyploid nuclear changes were more prominent in the CAH-B group than in the CAH-C group.
In conclusion, follicular portal inflammation and severe sinusoidal lymphocytic reactions were common histologic findings in serologically proven CAH-C when compared to CAH-B.
Since the development of the sensitive serological tests for the diagnosis of hepatitis C infection, many reports have been published about the prevalence, epidemiology and clinical characteristics of acute and chronic hepatitis C1–3). Before the development of this sensitive method detecting hepatitis C viral infection, non-A, non-B hepatitis could be diagnosed by careful history and checking the surrogate hepatitis viral markers4). Although it has been suggested that the most consistent histological features of non-A, non-B hepatitis are the formation of lymphoid follicles with germinal centers in the portal tracts and steatosis in the liver parenchyma, until now only a few reports on the histologic findings of chronic hepatitis C were published and characteristic histologic findings of chronic hepatitis C have been not yet determined5–6).
The aims of this study are to analyze retrospectively the histologic findings of the chronic active hepatitis C diagnosed serologically and to determine the presence of the characteristic differential points between chronic active hepatitis C and chronic active hepatitis B.
The subject population consisted of nineteen patients with chronic active hepatitis C (CAH-C) and nineteen patients with chronic active hepatitis B (CAH-B). The clinical characteristics of the subjects were summarized in Table 1. Diagnosis of chronic active hepatitis was made by peritoneoscopic liver biopsy with the compatible clinical and laboratory findings. Of those patients who had a peritoneoscopic liver biopsy between January 1989 and August 1990 in the Yonsei University College of Medicine, we selected the patients whose blood samplings were cryo-preserved and feasible for hepatitis viral markers study. The subjects were divided into two groups according to the results of the hepatitis viral markers. One group (CAH-C) was the 19 patients who had positive tests for Anti-HCV (EIA, Chiron Co., USA) and negative tests for HBsAg (RIA, Abbott Co., USA). The other group (CAH-B) was the 19 patients who had negative tests for Anti-HCV and positive tests for HBsAg. There were no differences in age, sex, and liver function tests between these two groups, but past medical history such as transfusion and acupuncture was found more frequently in CAH-C group (13 of 19 cases) than in CAH-B group (2 of 19 cases).
Three pathologists and two physicians reviewed the pathologic slides individually, without previous informations on the patients, and made scores according to the histologic activity index (HAI), modified from Knodell’s proposal7). Histological features were analyzed between the CAH-C and CAH-B groups in terms of portal inflammation (1. scattered, 2. diffuse, 3. follicular), periportal necroinflammation (1. expansile, 2. stellate, 3. bridging, 4. precirrhotic), focal necrosis or acidophilic body (1. 1/100×, 2. 2–3/100×, 3. 3/100×, 4. confluent), sinusoidal lymphocytic reaction (0. absent, 1. mild to moderate, 2. severe), regeneration (1. scattered, 2. diffuse cobble, 3. follicular), polyploid nuclear change (0. absent, 1. present), terminal hepatic vein fibrosis (0. absent, 1. perivenular or perihepatocellular), fatty change (0. absent, 1. mild, 2. moderate, 3. severe) (Table 2).
We used the Odds ratio and p value to compare the HAI between the CAH-C and CAH-B groups. Statistical significance was determined when the p value was below 0.05. Odds ratio, which is a classical index used to measure the association and strength of association between the disease and expression, was used to clarify arithmatically the association between the HAI and type of viral hepatitis.
The follicular portal inflammation with dense lymphocytic infiltrates was noted in ten cases of the CAH-C group (52.6%), whereas only in five cases of the CAH-B group (25.3%). There were statistically significant differences in the morphologic changes of portal inflammation between these two groups (Table 3, Fig. 1).
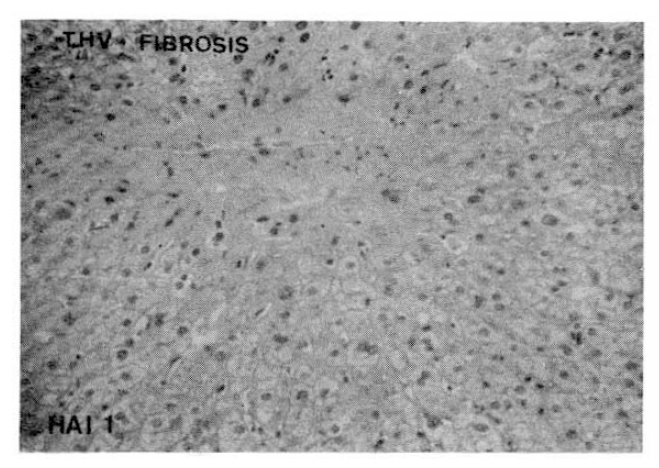
Higher indices of portal fibrosis, such as bridging or precirrhotic changes, were more prominent in the CAH-B group (68.4%) than in the CAH-C group (42.1%). Perivenular or perihepatocellular fibrosis was noted frequently in both groups (63.2% in CAH-C and 78.9% in CAH-B group respectively), but there was no significant difference in the morphologic feature of terminal hepatic vein fibrosis between these two groups (Table 5,6, Fig. 3,4,).
Although clinical and experimental evidence suggesting that etiologic agents of hepatitis C are virus-like in nature have accumulated in the last decade, and there have been intensive efforts in many laboratories throughout the world in the past few years to identify and characterize the agents responsible for this infection, no hepatitis C agents have been identifed as an antigenic, ultrastructural or molecular entity8). An almost 15-year quest to identify an agent of non-A, non-B (NANB) viral hepatitis ended in 1988 with the identification of a RNA virus with an immunologic specificity for transfusion associated NANB hepatitis with the molecular biological advances9–10). Eighty percent of patients with transfusion-associated NANB hepatitis and sixty percent with sporadic form, formerly presumptively suggested by checking surrogate hepatitis viral markers, have detectable anti-HCV antibody11). Thereafter, it has been determined that hepatitis C accounts for a substantial proportion of acute, chronic liver diseases, and hepatocellular carcinoma12). Further studies to detemine the roles of infections with hepatitis C virus to this leading cause of liver diseases are now underway worldwide13,14).
Although several histological patterns have been described in patients with acute and chronic NANB virus infection, none of them facilitates a definite histological diagnosis, and the criteria to be described must be regarded as suggestive rather than diagnostic15). Until now, the most consistent feature of NANB hepatitis has been the formation of lymphoid follicles with germinal centers in portal tracts, but these have also been found in some patients with chronic HBV infections16). Some characteristics such as steatosis in the parenchyma, giant cells, diffuse sinusoidal cells infiltrates and bile duct lesions have been emphasized as more or less indicative of NANB etiology of viral hepatitis17). The histological features of patients with sporadic NANB hepatitis were described by Bamber et al.18) Histologic findings in liver biopsy covered the whole spectrum of acute and chronic hepatitis. The notable features in these biopsies were the presence of fatty changes and excessive cellularity of sinusoids in relation to the degree of hepatic necrosis. The presence of considerable fatty change, particularly in the early weeks of acute hepatitis, was noteworthy because it has been regarded as unusual in other types of acute viral hepatitis. Dienes et al.19) studied the specimens from 57 patients with acute or chronic hepatitis NANB and found the two types of pathologic features. One was an eosinophilic alternation of the hepatocyte cytoplasm associated with many eosinophilic bodies. The second feature consisted of sinucoidal lymphocytic infiltrations. Both features were associated with portal and mild periportal inflammation unrelated to the degree of parenchymal reaction. Similar findings were also noted in our studies such as dense, compact portal lymphocytes infiltrations with expansions of portal tract and severe sinusoidal lymphocytic reactions.
The report about the histopatholgic comparison between 86 cases of hepatitis A and 76 cases of NANB hepatitis published by Krager and Christoffersen20) revealed that hepatitis A was charcterized by more pronounced portal inflammations than hepatitis NANB, but less conspicuous parenchymal changes such as focal nerosis, Kupffer cell proliferations, acidophilic bodies and ballooning change of hepatocytes. Steatosis occurred in 10% of hepatitis A compared with 26% in hepatitis NANB. Jove et al.21) analyzed retrospectively the clinical, morphological and evolutive features of 60 patients with chronic hepatitis NANB and found that there were some differences in morphological categories between sporadic and transfusion related cases. Takada et al.22) reported that there are at least two or more types of acute NANB hepatitis. In one type hepatocyte necrosis was prominent in the periportal area (portal type), and in the other there was no such characteristic distribution of hepatocyte necrosis (non-portal or diffuse type). Takase et al.22) also stressed that a histological analysis of the liver is important in order to distingish between the types of acute NANB hepatitis.
Recently, Patel et al.24) reviewed histological features in 24 cases of HCV antibody positive post-transfusion hepatitis and found that the majority of these biopsies showed portal, periportal and lobular inflammation with lymphoid follicles, considered to be characteristic of HCV liver disease. Govindarajan et al.25) also retrospectively analyzed pathologic features of 11 liver biopsies of chronic HCV infection. They concluded that serologically documented chronic HCV infection frequently exhibited morphologic features such as fat, sinusoidal collagen, glycogen in nuclei and atypical ducts, which are unsual for the other type of chronic viral hepatitis.
Beside the microscopic features described above, the ultrastructural findings in NANB were reported by many authors26–28). In NANB hepatitis, the liver ceil nuclei are characterized by irregular outlines, increased lobulization, thickening of the nuclear membrane, condensation and margination of the chromatin and intranuclear cytoplasmic inclusions. The most consistently reported finding is the presence of intranuclear aggregates. An overview of the various ultrastructural alternations reported in NANB hepatitis leads to the conclusion that the search for the morphologic components of this viral agent has not yet been completed.
Although there were no characteristic histologic features peculiar to chronic hepatitis C, dense, follicular portal lymphocytic inflammations and expansion with sinusoidal lymphocytic reactions seem to be more or less prominent histologic findings in diagnosing hepatitis C. However, further studies on the identification of hepatitis C virus and its characteristic histologic features will be continued.
Fig. 1.
Histopathologic appearance of portal inflammation, scattered (HAI 1, left) and follicular portal inflammation (HAI 3, right).
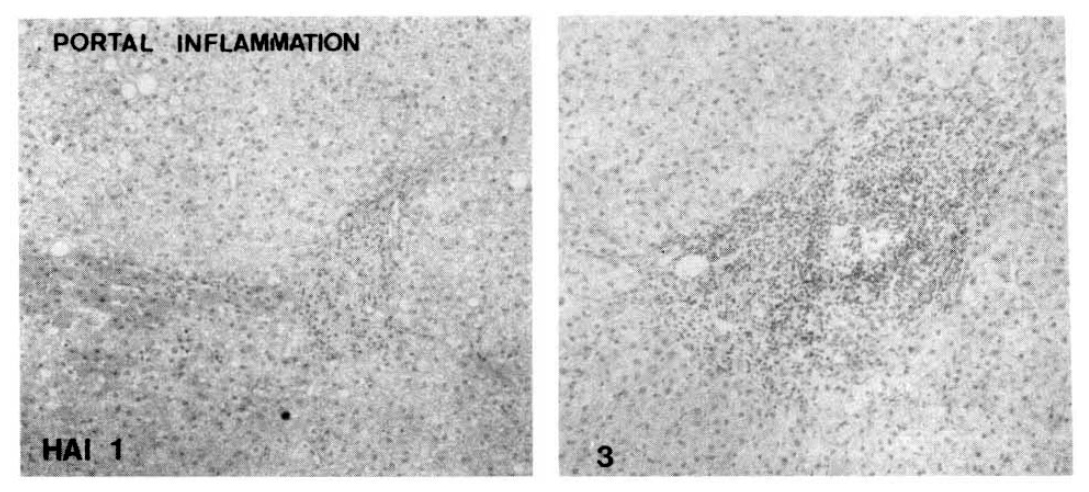
Fig. 2.
Histopathologic appearance of periportal necro-inflammation, spill-over (HAI 1, left) and bridging inflammation (HAI 4, right).
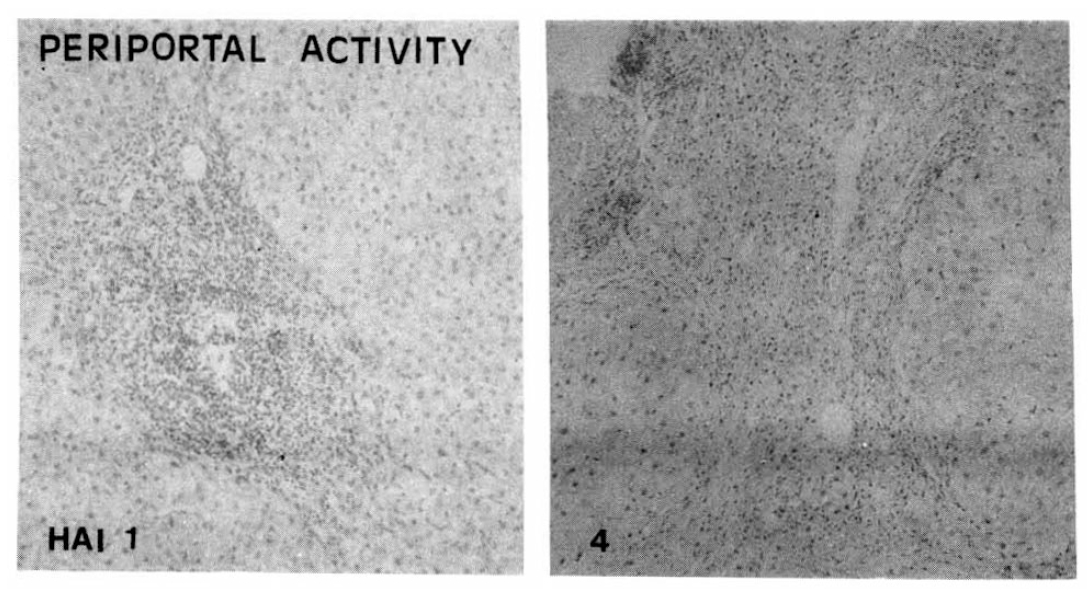
Fig. 3.
Histopathologic appearance of portal fibrosis, expansile (HAI 1, left) and precirrhotic portal fibrosis (HAI 4, right).
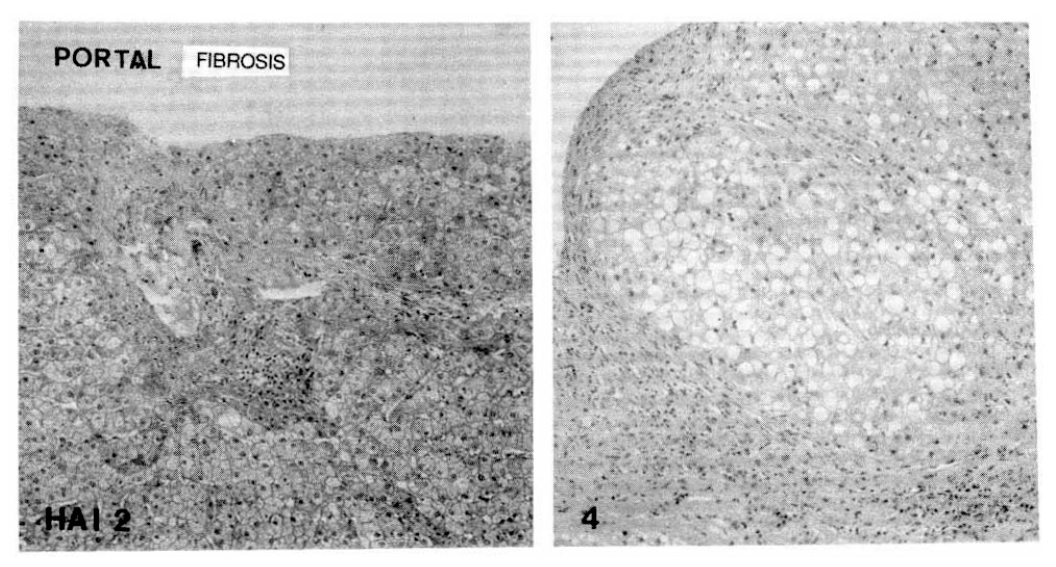
Fig. 5.
Histopathologic appearance of focal necrosis (vacant arrow) and acidophilic body (filled arrow).
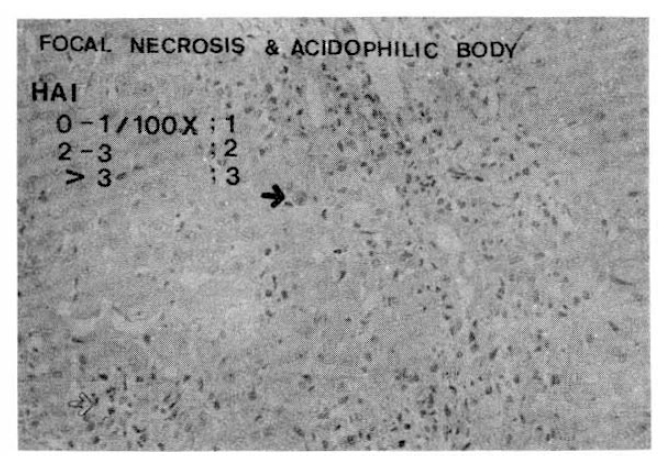
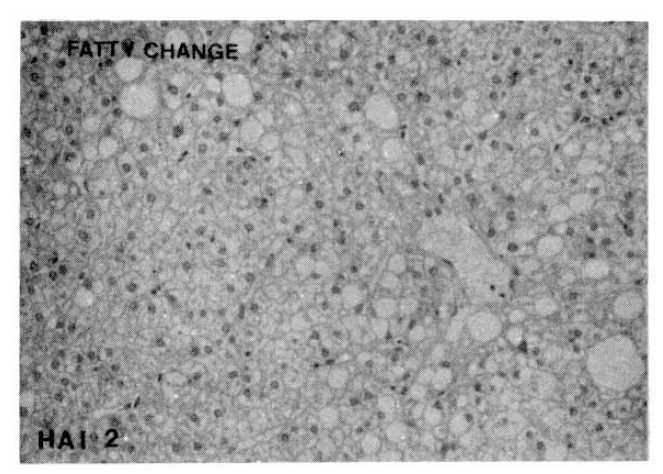
Fig. 6.
Histopathologic appearance of sinusoidal lymphocytic reaction, mild to moderate (HAI 1, left) and severe sinusoidal lymphocytic reaction (HAI 2, right).
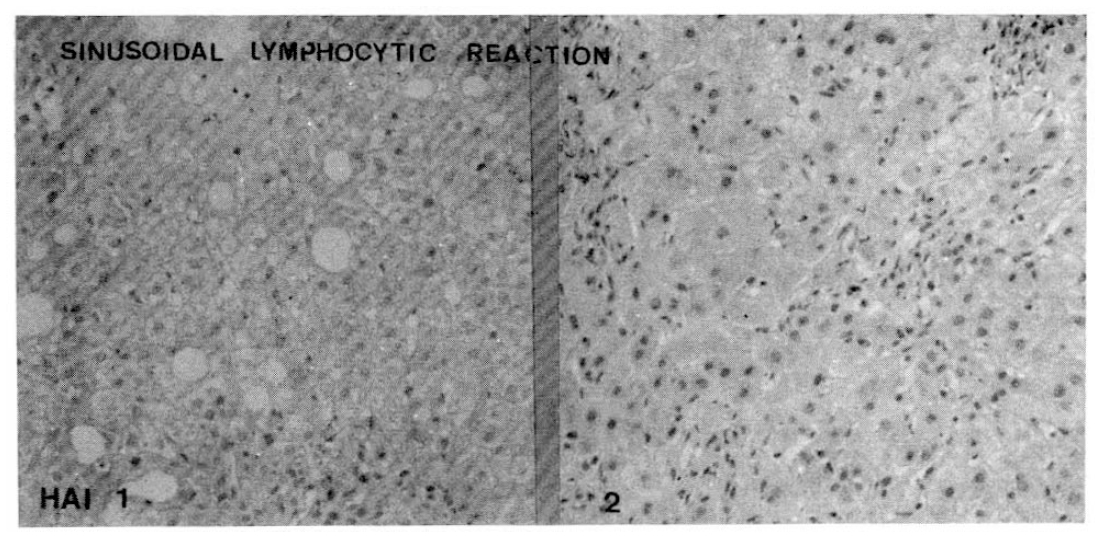
Fig. 9.
The follicular and severe portal inflammations (above fig.) with sinusoidal lymphocytic infiltrations (below fig.) were more prominent findings in chronic hepatitis C than chronic hepatitis B.
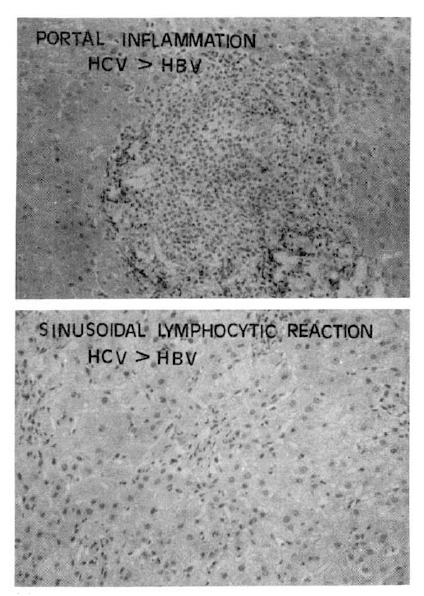
Table 1.
Clinical Profiles of Patients with Chronic Active Hepatitis
Table 2.
Histologic Assessment Indices (HAI)*
Table 3.
Portal Inflammation
| HAI | CAH-C | CAH-B |
|---|---|---|
| 1 (scattered) | 1 (5.3) | 6 (31.6) |
| 2 (diffuse) | 8 (42.1) | 8 (42.1) |
| 3 (follicular) | 10 (52.6) | 5 (25.3) |
Table 4.
Periportal Necroinflammation
| HAI | CAH-C | CAH-B |
|---|---|---|
| 1 (spill-over) | 10 (52.6) | 1 (5.3) |
| 2 (focal piecemeal) | 4 (21.1) | 3 (15.8) |
| 3 (multiple piecemeal) | 1 (5.2) | 7 (36.8) |
| 4 (bridging) | 4 (21.1) | 8 (42.1) |
Table 5.
Portal Fibrosis
| HAI | CAH-C | CAH-B |
|---|---|---|
| 1 (expansile) | 5 (26.3) | 0 (0.0) |
| 2 (stellate) | 6 (31.6) | 6 (31.6) |
| 3 (bridging) | 6 (31.6) | 11 (57.9) |
| 4 (precirrhotic) | 2 (10.5) | 2 (10.5) |
Table 6.
Terminal Hepatic Vein Fibrosis
| HAI | CAH-C | CAH-B |
|---|---|---|
| 0 (absent) | 5 (26.3) | 1 (5.3) |
| 1 (perivenular) | 12 (63.2) | 15 (78.9) |
| 1 (perihepatocellular) | 2 (10.5) | 3 (15.8) |
Table 7.
Focal necrosis or Acidophilic Body
Table 8.
Sinusoidal Lymphocytic Reaction
| HAI | CAH-C | CAH-B |
|---|---|---|
| 0 (absent) | 0 (0.0) | 1 (5.2) |
| 1 (mild/moderate) | 8 (42.1) | 12 (63.2) |
| 2 (severe) | 11 (57.9) | 6 (31,6) |
Table 9.
Regeneration
| HAI | CAH-C | CAH-B |
|---|---|---|
| 1 (scattered) | 6 (31.6) | 0 (0.0) |
| 2 (diffuse cobble) | 5 (26.3) | 1 (5.3) |
| 3 (follicular) | 8 (42.1) | 18 (94.7) |
Table 10.
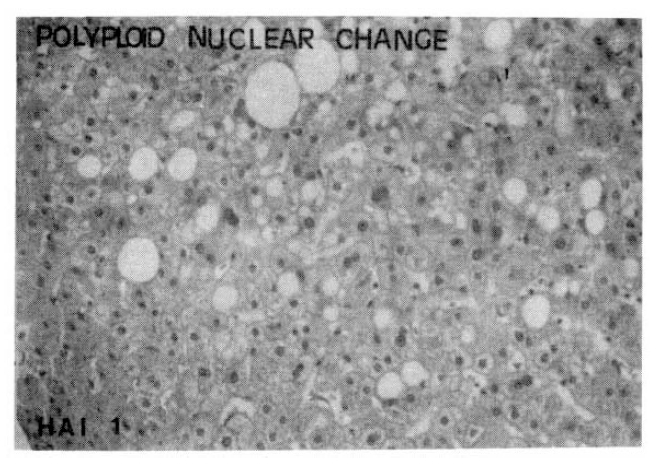
Polyploid Nuclear Change
| HAI | CAH-C | CAH-B |
|---|---|---|
| 0 (absent) | 10 (52.6) | 1 (5.3) |
| 1 (present) | 9 (47.4) | 18 (94.7) |
Table 11.
Fatty Change
| HAI | CAH-C | CAH-B |
|---|---|---|
| 0 (absent) | 11 (57.9) | 13 (68.4) |
| 1, 2 (mild/moderate) | 6 (31.6) | 6 (31.6) |
| 3 (severe) | 2 (10.5) | 0 (0.0) |
Table 12.
Odds Ratio of Each Index to Predict the Diagnosis
REFERENCES
1. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA derived from a blood-born non-A, non-B viral hepatitis genome. Science 244:359. 1989.


2. Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE, Tegtmeier GE, Bonino F, Colombo M, Lee SW, Kuo C, Berger K, Shuster JR, Overby LR, Bradley DW, Houghton M. An assay for circulating etiologic virus of human non-A, non-B hepatitis. Science 244:362. 1989.


3. Alter MJ, Coleman PJ, Alexander WJ, Kramer E, Miller JK, Mandel E, Hadler SC, Margolis HS. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. JAMA 262:1201. 1989.


4. Khuroo MS. Study of an epidemic of non-A, non-B hepatitis-possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med 68:818. 1980.


5. Mattsson L, Weiland O, Glaumann H. Long-term follow-up of chronic post-transfusion non-A, non-B hepatitis; Clinical and histological outcome. Liver 8:184. 1988.


7. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431. 1981.


8. Okamoto H, Okada S, Sugiyama Y, Yotsumoto S, Tanaka T, Yoshizawa H, Tsuda F, Miyakawa Y, Mayumi M. The 5′-terminal sequence of the hepatitis C virus genome. Japan J Exp Med 60:167. 1990.
9. Alter HJ, Purcell RH, Shih JW, Melpolder JC, Houghton M, Choo QL, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med 321:1494. 1989.


10. McFarlane IG, Smith HM, Johnson PJ, Bray GP, Vergani D, Williams R. Hepatitis C virus antibodies in chronic active hepatitis-pathogenetic factor or false-positive result? Lancet 335:754. 1990.


11. Kiyosawa K, Akahane Y, Nagata A, Furuta S. Hepatocellular carcinoma after non-A, non-B post-transfusion hepatitis. Am J Gastroenterol 79:777. 1984.

12. Colombo M, Choo QL, Ninno ED. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet 2:1006. 1989.


13. Arima T, Shimomura H, Nakajima T, Kanai K, Nagashima H, Tsuji T. Cloning of hepatitis C virus genomes and their properties. Gastroenterol Jpn 25:72. 1990.


15. Realdi G, Alberti A, Rugge M, Rigoli AM, Tremolada F, Schivazappa L, Ruol A. Long-term follow-up of acute and chronic non-A, non-B post-transfusion hepatitis; Evidence of progression to liver cirrhosis. Gut 23:270. 1982.



16. Okuno T, Shindo M, Arai K, Matsumoto M, Takeda M. Histological improvement of chronic hepatitis B, and non-A, non-B with interferon treatment; Application of a numerical scoring system for evaluating sequential morphologic changes. Gastroenterol Jpn 25:70. 1990.


17. Knodell RG, Conrad ME, Ishak KG. Development of chronic liver disease after acute non-A, non-B post-transfusion hepatitis. Gastroenterology 72:902. 1977.


18. Bamber M, Murray A, Weller I, Morelli A, Scheuer PJ, Thomas HC, Sherlock S. Clinical and histological features of a group of patients with sporadic non-A, non-B hepatitis. J Clin Pathol 34:1175. 1981.



19. Dienes HP, Popper H, Arnold W, Lobeck H. Histological observations in human hepatitis non-A, non-B. Hepatology 2:562. 1982.


20. Krager , Christoffersen P. Liver histopathology of hepatitis A virus infection; A comparison with hepatitis type B and non-A, non-B. J Clin Pathol 36:650. 1983.



21. Jove J, Sanchez-Tapias JM, Bruguera M, Mas A, Costa J, Barrera JM, Rodes J. Post-transfusional vs. sporadic non-A, non-B chronic hepatitis. A clinico-pathological and evolutive study. Liver 8:42. 1988.


22. Takada A, Nakaya H, Takase S. An outbreak of postoperative acute hepatitis due to non-A, non-B hepatitis virus. Jpn J Gastroenterol 78:856. 1981.
23. Takase S, Takada A, Sato H. A diagnostic approach to different types of non-A, non-B acute hepatitis through the evaluation of the lobular distribution of hepatocytic damage. Gastroenterol Jpn 25:61. 1990.


24. Patel AS, Sherlock S, Dusheiko G, Scheuer PJ, Ashrafzadeh P. Clinical course and histological correlations in serum hepatitis virus (HCV) antibody positive post-transfusion hepatitis. International symposium on viral hepatitis and liver disease the 1990 (abstract). 165.
25. Govindarajan S, Kanel GC, McHutchinson JG, Redeker AG, Kuo G. Morphologic features of chronic HCV infection-liver biopsy study. International symposium on viral hepatitis and liver disease the 1990 (abstract). 146.
26. Desment VJ, De Vos R. Ultrastructural findings in non-A, non-B viral hepatitis. Hepatology: A Festscrift for Hans Popper 1985;159.
-
METRICS

-
- 0 Crossref
- 2 Scopus
- 10,991 View
- 102 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


