 |
 |
| Korean J Intern Med > Volume 9(2); 1994 > Article |
|
Abstract
We report a case of acute herpetic esophagitis in a 33 year old man who was presumed to be immuno-compromised following prolonged steroid and cyclosporin treatment for acute rejection of a transplanted kidney. In Korea, all reported cases of herpetic esophagitis have been diagnosed in immuno-compromised and debilitated patients with a typical endoscopic appearance of ulcerating lesions. However, our patient showed multiple vesicular lesions without ulcer along the entire esophagus. The diagnosis was confirmed by colorimetric detection of herpes virus DNA using in situ hybridization.
The endoscopic findings reported herein probably represent the typical early stage of acute herpetic esophagitis.
The majority of herpetic esopagitis cases have historically been diagnosed at post-mortem examination of subjects who were immunocompromised or severely debilitated1ŌĆō3). However, with advances in diagnostic pracedures, many well documented cases of herpes esophagitis have also been reported in healthy subjects, as well as in immuno-compromised hosts4,5).
The diagnosis of herpetic esophagitis in most cases is suggested by histological findings of Cowdry type A intranuclear inclusions, and multinucleated giant cells. However, the exact nature of these cells cannot be determined by morphological appearance alone. Several specific diagnostic procedures have been used to confirm the diagnosis of herpetic esophagitis. These procedures include animal inoculation6), virus culture7), immuno-histochemistry8), electron microscopy9), and in situ hybridization of viral DNA10).
The most common endoscopic appearance of reported herpetic esophagitis is superficial punched-out ulcers which are frequently covered with fibrinous material. However, vesicular lesions are infrequently seen around the edges of ulcers11). These endoscopic findings are not sufficiently distinctive to differentiate herpetic esophagitis from esophagitis due to other causes, including other viruses, monilia, peptic disease, and radiation12). There have only been a limited number of reported cases diagnosed as herpetic esophagitis in which endoscopic findings showed small, discrete vesicles with erythematous bases13,14).
Our patient was presumed to be immunocompromised following prolonged steroid and cyclosporin therapy for acute rejection of a transplanted kidney. Endoscopic examination was performed because of epigastric discomfort. This is a case report of herpes esophagitis in what we believe is its earliest stage, based on endoscopic appearance.
A 33 year old man was admitted to the hospital with fever and decreased urine output. Thirty six days prior to admission, renal transplantation was performed in this hospital. He was placed on intravenous solumedrol pulse followed by maintenance oral prednisone and intravenous cyclosporin for 2 weeks. He complained of mild epigastric discomfort and nausea. His blood pressure was 140/90 mmHg, body temperature 37.5 ┬░C, pulse 88, and respirations 22. On physical examination the lungs were clear with a normal regular heart beats with out murmur. There was no organo-megaly, but mild tenderness and swelling of the operation site was noted.
Laboratory analysis revealed hemoglobin 9.9 gm/dl, hematocrit 29.4%, white blood cell 10,500/mm3 with normal differential and platelets 207,000/mm3. Blood chemistry showed FBS 125mg/dl, BUN 79.9mg/dl, Cr 7.1mg/dl, Na 129mM/L, K6.2mM/L, Ca 8.1 mg/dl, P 2.7mg/dl, Cl 92mM/L, total protein 5.0gm/dl, albumin 3.0gm/dl, total bilirubin 0.2gm/dl, AST 10 IU/L, ALT 22IU/L, alkaline phosphatase 77IU/L, total cholesterol 188mg/dl, and prothrombin time 11 sec. Urinalysis revealed protein+4 with many red blood cells under microscopy.
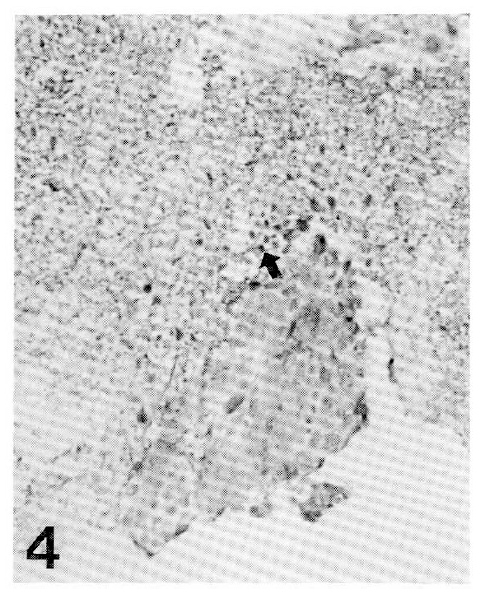
An endoscopic examination was performed on the 11th hospital day. There were multiple dark red, grouped and discrete vesicles of 3 to 5mm in diameter along the entire esophagus (Fig. 1,2). The stomach showed several flat erosions confined to the antrum, but the duodenum was normal. All vesicular lesions were easily removed from the esophagus with biopsy forceps. Light microscopy showed characteristic intranuclear inclusions and multi-nucleated giant cells (Fig. 3). The patient was placed on acyclovir from the 14th hospital day. Follow up endoscopy 11 days later revealed a complete absence of vesicles and the presence of faint scars in the esophagus. Renal impairment was also improved. For the confirmation of herpes esophagitis, in situ hybridization of herpes viral DNA was performed, resulting in a positive test for type 1 herpes simplex (Fig. 4).
In 1943, Pearce and Dagradi described viral incusions in acute ulcerative esophagitis at autopsy15). They presumed that these esophageal ulcers were caused by herpes virus. Almost all cases of herpetic esophagitis were diagnosed at autopsy before the emergence of fiberoptic endoscopy. However, technological advances in the endoscopic device and corresponding improvements in diagnostic procedures have resulted in easier and earlier diagnosis of herpetic esophagitis.
Herpes simplex is a DNA virus which involves visceral organs of immuno-compromised hosts. Of all the visceral organs, the esophagus has been reported as the most frequent infection site3,16). The reported prevalence of herpetic esophagitis has varied considerably with the method of analysis and the type of population studied. In autopsy series, the indicence of herpetic esophagitis was reported as 1.4 to 25%3,17). Although endoscopic diagnosis was first made by Weiden and Schuffler18), the prevalence of herpetic esophagitis in the general population is still unknown.
Symptoms frequently related to esophageal herpes have been described as odynophagia and abrupt onset of chest pain in 50 to 60% of patients, whereas no specific symtoms were presented in 26% of patients19). Gastrointestinal bleeding and unexplained nausea and vomiting were also reported.
Visceral herpes is commonly suspected on the bases of light microscopic findings of giant cells and Cowdry type A intranuclear inclusions in the tissue specimen, although these findings have also been attributed to other virus infections and nonviral diseases. Cytomegalovirus can cause viral inclusions in visceral organs. These inclusions are usually found in fibroblasts and endothelial cells, but not in epithelial cells. Because herpetic esophagitis is sometimes superinfected with cytomegalovirus and fungi, it is essential to make a rapid diagnosis of viral pathogens for a specific antiviral treatment. The method that offers the greatest sensitivity is tissue culture, but this method requires a long period of time7,20). Other types of analysis for clinical materials are more rapid. These methods include electron microscopy, detection of specific viral antigens using immuno-histochemical techniques, and routine histological examination for typical viral inclusions. However, the most recent analytical technique developed for viral identification is nucleic acid in situ hybridization. In this type of analysis, specific genetic information is obtained and a viral agent can be positively identified.
In situ hybridization was originally developed by Pardue and Gall10). Brigati et al21) demonstrated the technical feasibility of colorimetric in situ hybridization analysis of routinely fixed tissues embedded in paraffin. In this study, one of the most rapid techniques which requires less than 90 min was used22). However, it remains difficult to directly determine the sensitivity of in situ hybridization techniques for viral agent identification in prepared tissue sections. At a cost of a loss of sensitivity, in situ hybridization offers a more rapid specific identification of viral agents than viral tissue culture20).
The endoscopic appearance of herpetic esophagitis sometimes has an atypical pattern because the necrotic base becomes secondarily infected with bacteria and/or fungi3,19). However, the most frequent findings of herpetic esophagitis are ulcers in 80% of patients19). The size, shape, location, and appearance of these ulcers have no distinctive pattern3).
There have been several reports of herpetic eosphagitis in Korea23ŌĆō25). Endoscopic findings in these cases were typically ulcerating lesions. However, in one case, confirmed by in situ hybridization analysis of herpes virus DNA, endoscopic findings showed diffuse esophageal ulcers with necrotic bases rather than the vesicular lesions present in our patient25). In a series of herpetic esophagitis, McDonald et al26) showed that the most frequent ulcer pattern is diffuse and shallow ulcerations, especially in the distal half of the esophagus. Sometimes these ulcers are large (1 to 2 cm in diameter), with serpiginous borders. McBane19) endoscopically classified the typical patterns of ulcers as single or multiple, discrete or coalescent, and covered or not covered with pseudomembrane. He showed that ulcers tend to be coalescent in the lower esophagus and are usually covered with fibrinous exudate or pseudomembrane. Besides ulcers, simple mucosal inflammation has also been diagnosed as herpetic esophagitis. These lesions might be healed herpetic ulcers. However, only a minority of reported herpetic esophagitis cases showed vesicular lesions.
Although herpetic esophagitis is usually a selflimiting disease in healthy subjects and is uncommonly followed by significant sequelae16,17), it infrequently involves serious complications, especially when it affects immunologically compromised hosts, resulting in severe hemorrhage27) and perforation of the viscus28,29). Esophageal herpes is a systemic disease and usually deteriorates immunocompromised hosts in a short period.
Recently, with increasing organ transplantation and wide use of immunosuppressive treatments for various diseases, the risk of herpetic esophagitis is rapidly increasing. However, recent advances in both antifungal and antiviral treatments will increase the chances to cure this illness, even in immuno-compromised hosts, when the disease is diagnosed promptly.
Fig.┬Ā1.┬Ā2.
Endoscopy revealed multiple, well-circumscribed vesicular eruptions scattered along the entire esophagus and the lesions varied in size from 0.3cm to 0.5cm in diameter. These vesicular lesions were dark red, not coalescent, and easily removable by biopsy forceps without remnant ulcer.


REFERENCES
2. Agha FP, Lee HH, Nostant TT. Herpetic esophagitis: A diagnostic challenge in immunocompromised patients. Am J Gastroenterol 81:246. 1986.

3. Buss DH, Scharyi M. Herpes virus infection of the esophagus and other visceral organs in adults. Am J Med 66:457. 1979.


4. Depew WT, Prentice RSA, Beck IT, Blakeman JM, Dacosta LA. Herpes simplex ulcerative esophagitis in healthy patients. Am J Gastroenterol 68:381. 1977.

5. Deshmukh M, Shah R, McCallum RW. Experience of herpes esophagitis in otherwise healthy patients. Am J Gastroenterol 79:173. 1984.

7. Enders JF, Weller TH, Robbins FC. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science 109:85. 1949.


8. Landry ML, Mayo DR, Hsiung GD. The need for a rapid and accurate viral diagnosis. Pharmacol Ther 18:107. 1983.

9. Hsiung GD, Fong CKY, August M. The use of electron microscopy in diagnosis of Viral infection:An overview. Prog Med Virol 25:133. 1979.

10. Pardue ML, Gall JG. Molecular hybridization of radioactive DNA of cytological preparation. Proc Natl Acad Sci USA 64:600. 1969.



12. Eras P, Goldstein MJ, Sherlock P. Candida infection of the gastrointestinal tract. Medicine 51:367. 1972.


13. Howiler W, Goldberg HI. Gastroesophageal involvement in herpes simples. Gastroenterology 70:775. 1976.


14. Fishbein PG, Tuthill R, Kressel H, Freidman H, Snape WJ Jr. Herpes simplex esophagitis:A cause of upper-gastrointestinal bleeding. Dig Dis Sci 24:540. 1979.


15. Pearce J, Dagradi A. Acute ulceration of the esophagus with associated intranuclear inclusion bodies:Report of four cases. Archives of Pathology 35:889. 1943.
16. Moses HL, Cheatham WJ. The frequency and significance of human herpetic esophagitis: An autopsy study. Laboratory investigation 12:663. 1963.

17. Nash G, Ross JS. Herpetic esophagitis: A common cause of esophageal ulceration. Hum Pathol 5:339. 1974.


18. Weiden PL, Schuffler MD. Herpes esophagitis complicating HodgkinŌĆÖs disease. Cancer 33:1100. 1974.


19. McBane RD, Gross JB Jr. Herpes esophagitis: Clinical syndrome, endoscopic appearance and diagnosis in 23 patients. Gastrointest Endosc 37:600. 1991.


20. Unger ER, Budgeon LR, Myerson D, Brigati DJ. Viral diagnosis by in situ hybridization: Description of a rapid simplified colorimetric method. Am J Surg Pathol 10:1. 1986.

21. Brigati DJ, Myerson DL, Leary JJ, Spalholz B, Travis SZ, Fong CKY, Hsuing GD, Ward DC. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotinlabeled hybridization probes Virology 50:39. 1983.
22. Iezzoni JC, Kang JH, Montone KT, Reed JA, Brigati DJ. Colorimetric detection of herpes simplex virus by DNA in situ sandwich hybridization: A rapid, formamidefree, random oligomerenhanced method. Nucleic Acid Res 20:1149. 1991.

23. Kim MJ, Cho KW, Lee S, Choi SK, Rew JS, Choi KC, Kim SJ, Yoon CM. Herpetic esophagitis in a renal transplant patient. Kor J Gastrointest Endosc 10:301. 1990.
24. Yim DS, Chung JB, Kim WH, Lee SI, Kang JK, Park IS, Choi HJ, Lee MG. A case of herpes esophagitis confirmed by electron microscopic findings. Kor J Gastrointest Endosc 11:73. 1991.
25. Yoon SK, Han IS, Lee SH, Park YH, Baek SH, Nam SM, Park HC, Whang IS, Chu HK, Kim JH. A case of herpes esophagitis confirmed by in situ hybridization. Korean J Int Med 14:122. 1993.
26. McDonald GB, Sharma P, Hackman RC, Meyers JD, Thomas ED. Esophageal infections in immunosuppressed patients after marrow transplantation. Gastroenterology 88:1111. 1985.


27. Rattner HM, Cooper DJ, Zaman MB. Severe bleeding from herpes esophagitis. Am J Gastroenterol 80:523. 1985.

-
METRICS

-
- 1 Crossref
- 0 Scopus
- 9,165 View
- 90 Download
- Related articles
-
Topical Steroids to Treat Granulomatous Mastitis: A Case Report2011 September;26(3)
Right Atrial Maass Associated with Hepatoma -2 Case Reports-1994 July;9(2)
Isolated Left Coronary Ostial Stenosis -A Case Report-1990 January;5(1)
Alveolar Hemorrhage Associated with Crescentic Glomerulonephritis -A Case Report-1989 January;4(1)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


