 |
 |
| Korean J Intern Med > Volume 20(3); 2005 > Article |
|
Abstract
In the era before reperfusion therapy, ventricular septal rupture complicated 1~3% of acute myocardial infarctions (AMI) usually 3-5 days after onset. Studies have reported a positive correlation between the incidence of septal perforation and total occlusion of the coronary arteries. A 70-year old female patient was referred to the emergency room with the diagnosis of acute anterior myocardial infarction (MI) and recent cerebral infarction. The coronary angiogram showed a 90% stenosis at the mid-portion of the left anterior descending artery (LAD), and the lesion was successfully treated by percutaneous coronary intervention (PCI) with stent implantation. After PCI, the anterior wall motion improved on the follow-up echocardiogram. However, on the 20th hospital day, the patient condition deteriorated suddenly with pulmonary congestion. The echocardiography revealed a 1.3 cm ventricular septal defect at the apical septum with a left-to-right shunt. We report this rare case of delayed septal rupture in a patient with patent LAD after PCI and recovery of wall motion.
A ventricular septal rupture (VSR) is a lethal complication of AMI. After VSR was first described by Latham in 1874, there had been steady increase of reported case. However, the development of reperfusion therapy has contributed to the reduction in incidence of VSR. The event usually occurs 3 to 5 days after AMI and often precipitates into cardiogenic shock, and early surgical treatment is necessary to reduce risk of fatality. We report a case of medically-treated VSR developed 16 days after PCI in a patient with acute anterior MI.
A 70-year-old female had chest pain for three days prior to admission as well as hemiplegia and dysarthria for 5 days. She was referred to our hospital for aggravating chest pain. At the previous hospital, she was admitted for the treatment of type 2 diabetes mellitus and tentorial ischemia. Four years ago, she was diagnosed as mucinous ovarian cystadenoma and underwent bilateral oophorectomy and total hysterectomy. She has no history of smoking or alcohol use. Her father had a history of cardiogenic shock.
On admission, the pulse was 112 beats per minute, the respiration 20 per minute, the blood pressure 120/70 mmHg and the body temperature 36℃. The patient was alert at the time of admission. However, she appeared to be acutely ill, and presented with pale conjunctiva and non-icteric sclera. There was no jugular vein distension, and the heart rhythm was irregular without S3 or heart murmur. The lung was clear without rale or crackles. The level of initial CK was 282 IU/L (normal 0~185), CK-MB was 19.2 ng/mL (normal 0~5.0), and TnT was 1.93 ng/mL (normal 0~0.01), and these levels decreased following admission. Hs-CRP level was 2.88 mg/dL (normal 0~0.4). WBC count was 15,800/µL (normal 4000~10,000), and neutrophil count 78.9% (normal 42.2~75.2%). The glucose level was 279 mg/dL and LDL-cholesterol level 157 mg/dL. Other blood chemical and enzyme levels were normal. The urine was normal. On the initial electrocardiogram, 3 mm ST segment elevation in leads V2 through V4 and Q wave in lead II, III, and aVF was observed (Figure 1A). The chest X-ray revealed no abnormalities (Figure 2A). On transthoracic echocardiography, the estimated left ventricular ejection fraction was 45%. There was severe hypokinesia of the cardiac apex and along the inferior wall with moderate pulmonary hypertension (right ventricular systolic pressure was estimated as 55 mmHg). No mural thrombus was seen. She was given conservative care with medications consisting of heparin, IV nitrate, clopidogrel, and aspirin.
On the fourth inpatient day, coronary angiography and PCI was performed. From the coronary angiography, 70% and 90% stenosis was observed on the proximal and mid--portion of the LAD, respectively. Ninety percent stenosis on the 2nd obtuse marginal branch was also observed (Figure 3A). PCI with stent implantation for LAD lesions was successful (Figure 3B).
On the seventh inpatient day, the patient experienced dyspnea but her heart rhythm was regular and no cardiac murmur was heard. The lung sounds were not coarse but fine crackles were heard at both lung bases. The chest radiograph revealed pulmonary congestion on both sides (Figure 2B). The patient was treated with diuretics, and she managed well.
On day 20, her heart rhythm was regular but a grade 4/6 pansystolic murmur was heard at the mid-left sternal border. Echocardiography revealed improved wall motion of the anterior wall and the cardiac apex, but a ventricular septal defect approximately 1.3 cm in diameter was observed at the apical septum (Figure 4). Because she did not show any serious symptoms related to the ventricular septal rupture and was nearly bed-ridden due to right hemiplegia as a sequel of cerebral infartion, only medical treatment was done. One month after discharge, she complained of dyspnea, and the chest radiograph showed pulmonary congestion with bilateral pleural effusion, but transthoracic echocardiography revealed normal wall motion of the cardiac apex and no change in the size of the defect. The diuretic dose was adjusted and she managed well. After 11-months of medical therapy, she died of an unknown cause.
The incidence of septal rupture1), decreased with the advent of reperfusion therapy since 1980's, prior to which septal rupture was reported to complicate 1 to 3 percent of AMI. VSR usually occurs within the first 10 to 14 days when necrotic tissue is abundant and the collateral circulation is not well developed2).
In recent studies, the mean onset time of VSR was reported to be about 4 days (range 1~19 days) after AMI, and thrombolytic therapy was shown to shorten the onset time of VSR3-5). In the case of primary angioplasty, VSR rarely occurred. Reviewing the literature, David et al. reported one case of septal rupture occurring on the 19th day after AMI out of 45 patients diagnosed as VSR after AMI from 1987 to 19956).
Risk factors for septal rupture include hypertension, advanced age, being female, the absence of smoking, the absence of a history of angina or MI, extensive MI, and right ventricular infarction1). In angiographic patterns, total occlusion of coronary arteries are more common. Although thrombolytic therapy prevents extensive transmural necrosis, thrombolysis also induces myocardial hemorrhage during the lytic state and precipitates the process of ventricular septal perforation.
In anterior AMI, loss of apical contraction may result in a further decrease in the apical wall thickness in systole, increasing tensile stress per unit cross-sectional area of the apical wall. That suggests mechanical factors in the development of early-phase rupture. Becker and van Mantgen7) have classified cases of cardiac rupture into 3 types, and cases of VSR are similar to those of cardiac rupture. Type I is characterized as an abrupt, slit-like myocardial tear correlating clinically with a recent infarction (<24h); type II has an area of myocardial erosion, indicative of a slowly progressive tear; and in type III, a fatal tear typically occurs where an aneurysm has formed.
After PCI, ischemia of the infarcted myocardium might be improved. However, residual ischemia itself and reperfusion injury may delay the recovery of infarcted myocardial function. The difference of hemodynamic influence between the infarcted zone and the border zone may play an important role on VSR.
We didn't confirm whether myocardial salvage of tissue level was successful through other imaging studies. However, the echocardiography showed improved apical wall motion suggesting that myocardial salvage of tissue level was successful since non-viable myocardium cannot contract.
After Cooley et al. performed the first successful surgical repair of VSR, early surgical repair has helped to lower mortality rates and improve survival. Medication use to stabilize the patient's condition is only a temporary option, because most patients rapidly deteriorate and die. Most patients require surgical intervention.
The mortality rate among patients with septal rupture who are treated conservatively without surgical closure is approximately 24 percent in the first 24 hours, 46 percent at one week, and 62 to 82 percent at two months8, 9).
VSR can develop after 30 days after AMI, but it happens rarely. We report this case since there have been no reported case of delayed ventricular septal rupture, which developed after the improvement of wall motion following PCI.
References
1. Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS, Vahanian A, Califf RM, Topol EJ. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation 2000;101:27–32PMID : 10618300.


2. Skehan JD, Carey C, Norrell MS, de Belder M, Balcon R, Mills PG. Patterns of coronary artery disease in post-infarction ventricular septal rupture. Br Heart J 1989;62:268–272PMID : 2803872.



3. Thiele H, Lauer B, Hambrecht R, Boudriot E, Sick P, Niebauer J, Falk V, Schuler G. Short- and long-term hemodynamic effects of intra-aortic ballon support in ventricular septal defect complicating acute myocardial infarction. Am J Cardiol 2003;92:450–454PMID : 12918526.


4. Rhydwen GR, Charman S, Schofield PM. Influence of thrombolytic therapy on the patterns of ventricular septal rupture after acute myocardial infarction. Postgrad Med J 2002;78:408–412PMID : 12151656.



5. Kinn JW, O'Neill WW, Benzuly KH, Jones DE, Grines CL. Primary angioplasty reduces risk of myocardial rupture compared to thrombolysis for acute myocardial infarction. Cathet Cardiovasc Diagn 1997;42:151–157PMID : 9328698.


6. David TE, Dale L, Sun Z. Postinfarction ventricular septal rupture: repair by endocardial patch with infarct exclusion. J Thorac Cardiovasc Surg 1995;110:1315–1322PMID : 7475183.


7. Becker AE, van Mantgem JP. Cardiac tamponade: a study of 50 hearts. Eur J Cardiol 1975;3:349–358PMID : 1193118.

Figure 1
The initial ECG shows 3 mmV ST elevation in V2,3,4 (A). The 1 day follow-up ECG shows decreased ST elevation in V2,3,4 (B).

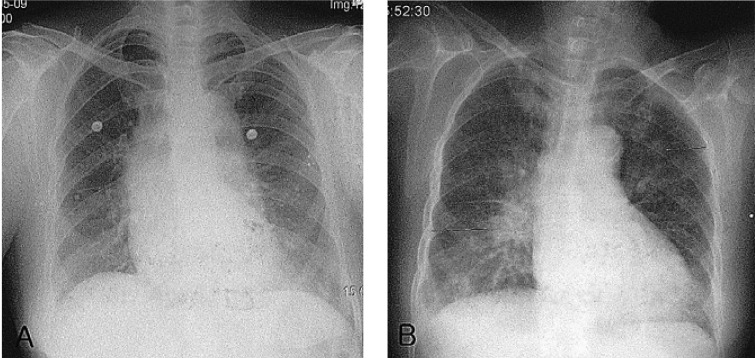
Figure 2
On admission day, there is no active lung lesion in the chest PA (A). On the 7th hospital day, there is pulmonary congestion (B).





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



