 |
 |
| Korean J Intern Med > Volume 26(2); 2011 > Article |
|
Abstract
Background/Aims
Increased osteoclast activity is a pivotal finding in osteoporosis. This increase is mediated via the mevalonate-to-cholesterol pathway, which is involved in producing the intermediates required for osteoclast activity. D-003, a mixture of high molecular weight sugarcane wax acids, has been shown to inhibit cholesterol synthesis prior to mevalonate production, resulting in a reduction of bone loss and resorption in ovariectomized rats. Moreover, previous studies have demonstrated that short-term D-003 treatment reduces urinary excretion of deoxypyridinoline/creatinine in postmenopausal women.
Methods
We performed a double-blinded, placebo-controlled study to investigate the effects of D-003 (10 mg/day) treatment for 3 years on bone mineral density (BMD) in 83 postmenopausal women with low BMD.
Results
Over 3 years, D-003 treatment increased lumbar spine BMD (5.1%, p < 0.01) and improved osteoporosis-related quality of life scores as compared with placebo-treated controls. D-003 was also well tolerated; the frequency of adverse events in the bone, joints, or muscle with D-003 treatment (p < 0.05) was lower than in the placebo group.
Osteoporosis is a chronic degenerative systemic disease characterized by low bone mineral density (BMD) and microarchitectural deterioration of bone tissue, which leads to increased bone fragility and fracture risk [1,2]. The incidence of osteoporosis increases with age and disproportionately affects older women and men, with postmenopausal osteoporosis being the most frequent type of primary osteoporosis [1,2].
A continuous bone remodeling process that involves the balance between bone resorption and bone formation maintains the integrity of the mature adult skeleton. This remodeling process is regulated by osteoclasts and osteoblasts, respectively. The imbalance of these processes in favor of the former leads to osteoporosis [3].
N-bisphosphonates, which are first-line antiosteoporotic drugs, exhibit their antiresorptive effects through the reduction of osteoclast activity, diminished recruitment and differentiation of osteoclast precursors, and enhanced osteoclast apoptosis [4]. The effects of N-bisphosphonates are due to the inhibition of farnesyl pyrophosphate synthase activity, an enzyme in the mevalonate pathway. This inhibition leads to a reduction in the formation of isoprenoid intermediates involved in the prenylation of guanine triphosphatases (GTP)-binding proteins, a process necessary for osteoclast membrane ruffling and bone resorptive functions [4-6].
All bisphosphonates are effective for treating osteoporosis. Bisphosphonate therapy results in modest gains in BMD and clinically relevant reductions in vertebral fracture rates [4-13]. Effects on hip and non-vertebral fracture risk, however, have been variable [12,13].
There is growing evidence that hyperlidemia and lipid oxidation contribute to both atherosclerosis and osteoporosis development as the products of lipid oxidation cause promotion and inhibition of osteoblastic differentiation in vascular and bone cells, respectively [14]. Consistent with these observations, competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase (statins) reduce the downstream metabolites of the mevalonate pathway, thus impairing the prenylation of GTPases and osteoclastic bone resorption. These inhibitors also induce bone formation through an increase in the expression and synthesis of bone morphogenetic protein (BMP) 2 [15]. Clinical studies have reported that statins increase BMD and reduce fracture rates; however, randomized controlled trials have failed to find protective effects of statins against fractures [16-18].
D-003 is a mixture of higher aliphatic acids purified from sugar cane wax, wherein octacosanoic (C28) acid is the most abundant, and C24, C25, C26, C27, C29, C30, C31, C32, C33, C34, C35, and C36 acids are at lower concentrations [19].
D-003 has been shown to inhibit mevalonate formation by regulating the activity of HMG CoA reductase [20], leading to the production of cholesterol-lowering effects [21-23] and the inhibition of lipid peroxidation [22,24].
Based on these observations, the effects of D-003 on experimentally induced osteoporosis were investigated. D-003 treatment has been shown to increase osteoclast apoptosis, thus preventing bone loss and bone resorption in rats with ovariectomy- [25-27] or prednisolone-induced [28] osteoporosis. In addition, D-003 treatment (10 mg/day) for 6 months reduced urinary excretion of deoxypyridinoline/creatinine (DPD/Cr), a bone resorption marker, in postmenopausal women [29].
The current study investigated the effects of D-003 treatment (10 mg/day) administered for 3 years on the BMD of the lumbar spine and femoral neck in postmenopausal women with low BMD.
This study was conducted in the Orthopedic Unit of the Surgical Medical Research Centre (Havana, Cuba) in accordance with the principles of the Helsinki Declaration and the Cuban Guidelines of Good Clinical Practices. The study protocol was approved by the institutional ethics and scientific board.
Postmenopausal women were enrolled after obtaining their informed written consent (Visit 1). A medical history, physical examination, and interview regarding osteoporotic risk factors were performed. All enrolled women underwent a placebo-baseline period for 2 weeks, in which blood samples for laboratory determinations were taken, and lumbar spine (L1-L4) and femoral BMD were measured. Hypercholesterolemic women were encouraged to continue or start a low-fat, low-cholesterol diet during the study. Eligible women were randomized (Visit 2), under double-blind conditions, to the placebo or D-003 (10 mg/day) groups for 3 years and attended visits after 1.5, 3, 6, and 12 months of treatment during the first year, and every 6 months thereafter (Visits 3-10). Physical examination, drug compliance, and adverse experience (AE) controls were also performed during the trial. Laboratory tests and quality of life interviews were performed at baseline and after 3, 6, and 12 months of treatment, and annually thereafter. BMD measurements were recorded at baseline and at yearly intervals.
We enrolled women (40 to 70 years) with amenorrhea of at least 12 months and ≥ 2 risk osteoporosis factors (personal or family history of fractures, low dietary calcium intake, physical inactivity, cigarette smoking, small, and thin frame; Caucasian or Asian race, excess of alcohol drinking, consumption of corticoids, and/or thyroid medications). Participants were eligible for randomization if they had a lumbar spine BMD value of at least 1 SD below mean normal peak levels provided by the densitometer manufacturer. This study, therefore, included both osteopenic and osteoporotic women.
Uncontrolled hypertension or diabetes, relevant renal or hepatic diseases, diagnosed neoplasias or diseases known to affect bone metabolism, such as hypothyroidism, hyperthyroidism or hyperparathyroidism, and clinically relevant vitamin D deficiency were categorized as exclusion criteria. Also, we excluded women who had unstable angina, myocardial infarction, stroke, or any major surgery within the 6 months prior to the trial. Those treated with bisphophonates, estrogens, glucocorticoids, fluoride, or calcitonin during this time were also excluded. Sixty-five women (78.3%) who had low dietary calcium consumption were advised to increase calcium-rich food (milk, yogurt, cheese, and beans) in their diet.
Eligible women were randomized, in a double blind fashion, to D-003 (10 mg/day) or placebo groups and were instructed to take the tablets once a day with the evening meal. The dosage of D-003 was chosen according to previous data in postmenopausal women [30].
Treatments were randomized in blocks of 10 (1:1 randomization ratio) by a computer-generated code. Treatment packages were labeled with the allocation code, which was revealed only if serious adverse events occurred during the trial. Participants and all study staff, except those who generated the allocation code, remained blinded to treatment allocation during the trial.
Drug compliance was assessed through counts of the remaining tablets and interviews. Bisphophonates, estrogens, glucocorticoids, fluoride, calcitonin, androgens, thyroid hormones, and drugs inhibiting cholesterol synthesis were prohibited from enrollment to study completion.
Increase in lumbar spine BMD as compared with the placebo was the primary end point. Increases in femoral neck BMD and osteoporosis-related quality of life domains were secondary endpoints. Lipid profile changes were collateral efficacy variables.
Patients underwent measurements of lumbar spine density (PA L1-L4 and lateral L3) and femoral neck density in a dual X-ray absorptiometry in a DPX-IQ Lunar DXA (Ontario, Canada), version 4.6 B. Quality control of dual-energy X-ray absorptiometry included technician training and certification, daily scanning of a lumbar spine phantom, and review of a random sample of scans. The in vitro coefficient of variation was ≤ 0.5%. The T scores were calculated using the normal women database provided by the manufacturer. BMD assessments were performed at baseline and annually.
The effects on quality of life (QL) were assessed using the questionnaire Qualeffo-41, which was validated for patients with vertebral fractures and included 41 questions associated with the domains of pain, physical function, social function, general health perception, and mental function [30].
Blood samples were collected after 8-10 hours of overnight fast. Serum total cholesterol (TC) and triglycerides (TG) were determined through enzymatic methods with reagent kits from Roche (Basel, Switzerland). High-density lipoprotein-cholesterol (HDL-C) was determined as the cholesterol content in the supernatant obtained after β-lipoproteins precipitation [31]. Low-density lipoprotein-cholesterol (LDL-C) values were calculated using the Friedewald equation [32]. Blood biochemistry safety indicators were determined with enzymatic methods using reagent kits from the same supplier. Analyses were done in a Hitachi 719 autoanalyzer (Tokyo, Japan) at the Medical Surgical Research Centre. Hematological parameters were determined using the automatic Hematological Complex equipment.
We performed a systematic quality control that controlled the accuracy and precision (within and between-day variations) of the methods.
An AE was defined as any undesirable experience that occurred during the study, regardless of whether it was treatment-related or not. All information on AEs was gathered at each patient contact. Women were instructed to report any unwanted signs, symptoms, or injuries that occurred during the trial. Since potential fractures were not efficacy outcomes, they were included in the AE analysis, and were identified by self-report and confirmed by radiology or surgical reports.
According to their intensity, AEs were classified as mild, moderate, or serious. Mild AEs were those that did not require specific treatment and/or stopping of study medication. Moderate AEs were those requiring specific treatment and/or withdrawal of study medication, and serious AEs were those leading to hospitalizations or death.
Safety indicators included physical (body weight, heart rate, blood systolic, and diastolic pressure), hematological (hemoglobin, hematocrit, red blood cells, white blood cells, and platelet counts), and blood biochemistry (alanine aminotransferase, aspartate aminotransferase, creatine phosphokinase, glucose, and creatinine) variables.
A sample size of 40 women/treatment group was expected to provide 80% power to detect a 3.0% between-group difference in the mean percent change from baseline in lumbar BMD. Data were analyzed on an intention to treat basis, including all randomized subjects. No interim analyses were performed.
Continuous variables were analyzed using a Wilcoxon test for matched samples (within group comparisons), and a Mann Whitney U test (between group comparisons). The significance of repeat comparisons was adjusted with the Bonferroni test [33], and results were confirmed by an analysis of variance (ANOVA). Categorical variables were compared with the Fisher's exact probability. All statistical analyses were performed using statistics data analysis software from windows.
Eighty-three of the 102 enrolled women were randomly assigned to either the D-003 or placebo group. Nineteen women were not eligible for the study due to the absence of osteoporosis risk factors (10) or BMD values higher than those required (9).
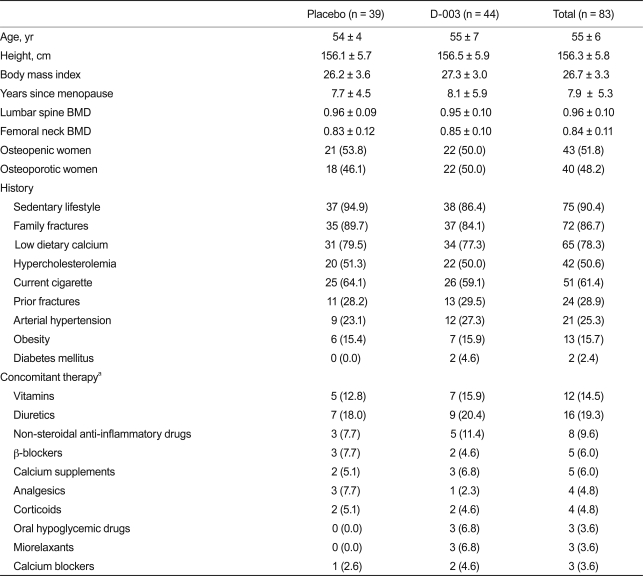
Table 1 lists the baseline characteristics of study subjects, which were statistically similar. The frequency of risk factors for osteoporosis was high and well balanced in the two study groups. The most common risk factor (> 50%) was postmenopause (100%), an enrollment requisite; followed by sedentary lifestyle (90.4%), low calcium intake (78.3%), family history of fractures (86.7%), and current smoking (61.4%). Participants had a mean age of 55 years. Study subjects also had a relatively high frequency of coronary risk factors other than smoking and postmenopausal status, including hypercholesterolemia and hypertension.
Patients did not consume drugs aimed at improving lipid and/or bone metabolism. The distribution of concomitant therapies was similar in both groups.
Subjects were followed for 3 years, and 18/83 subjects (21.7%; 8 placebo, 10 D-003 treated) withdrew prematurely from the trial, four of them due to AE. The AEs included two placebo patients who experienced spine surgery and right abdominal pain, respectively, and two D-003-treated women with heartburn and nausea, respectively. The other discontinuations were due to unwillingness to perform follow-up, protocol violations, and travel abroad. The withdrawal rate in the two groups was similar.
Excluding the premature withdrawals, compliance with the study medication was very good and well matched in both groups.
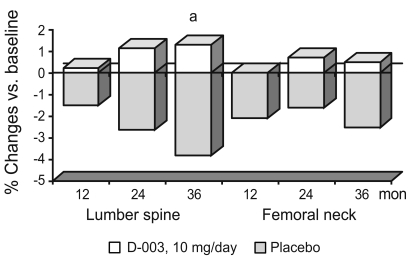
Fig. 1 summarizes BMD values and percent changes in baseline values, respectively. After completing 1, 2, and 3 years of treatment, lumbar spine BMD (average of L1-L4) progressively decreased by 1.5%, 2.6%, and 3.8% in the placebo group (p < 0.001 vs. baseline), whereas it increased by 0.2%, 1.1%, and 1.3% in the D-003-treated group. As compared with the placebo group, D-003 treatment significantly (p < 0.01) increased average lumbar spine BMD (difference vs. placebo, 5.1%).
The mean BMD of the femoral neck in the placebo group after 1, 2, and 3 years of therapy declined significantly by 2.1%, 1.6%, and 2.5%, respectively, as compared with baseline. In contrast, mean BMD of the femoral neck was unchanged in the treated group (+0.02%, +0.7%, +0.5%). At the end of the study the mean BMD of the femoral neck in the D-003-treated group had increased by 3.0% as compared with the placebo group; however, this difference was not significant.
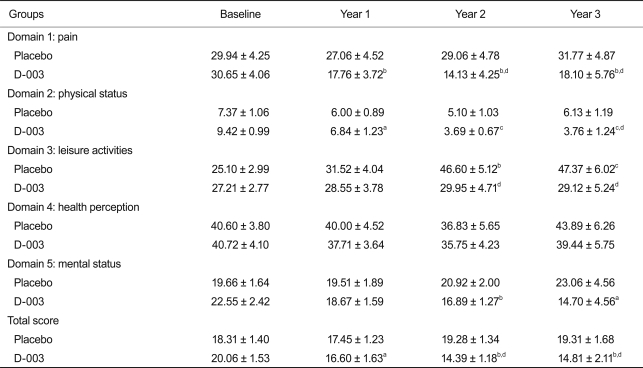
While no changes in osteoporosis-related quality of life domains were found in the placebo group, significant improvements in pain, physical status, and the total scores were seen in the D-003 group. Leisure activity values at 2 and 3 years for the placebo group were significantly lower than in the D-003 group. This observed decrease resulted from the significant deterioration of these values in the placebo group (Table 2). Other domains of the questionnaire were not significantly altered by the treatment.
Table 3 summarizes the effects on serum lipid profile variables, which were well matched in both groups at randomization. No lipid variables were significantly changed in the placebo group throughout the trial. After completing 3 years of therapy, D-003 treatment (10 mg/day) significantly decreased (p < 0.0001 vs. baseline and placebo) serum LDL-C (29.3%) and TC (14.7%), whereas HDL-C (25.6%) was significantly increased (p < 0.01 vs. baseline, p < 0.0001 vs. placebo). TG values, however, were unchanged with D-003 treatment.
The treatment was well tolerated. As mentioned previously, there were no significant differences between treatment groups in the number of patients who had withdrawn from therapy due to AE (2 from each group).
Periodic laboratory and physical safety indicator assessments revealed no differences between the D-003 and placebo groups. Additionally, individual values remained within normal limits, indicating that long-term D-003 administration did not impair the safety indicators assessed during the study.
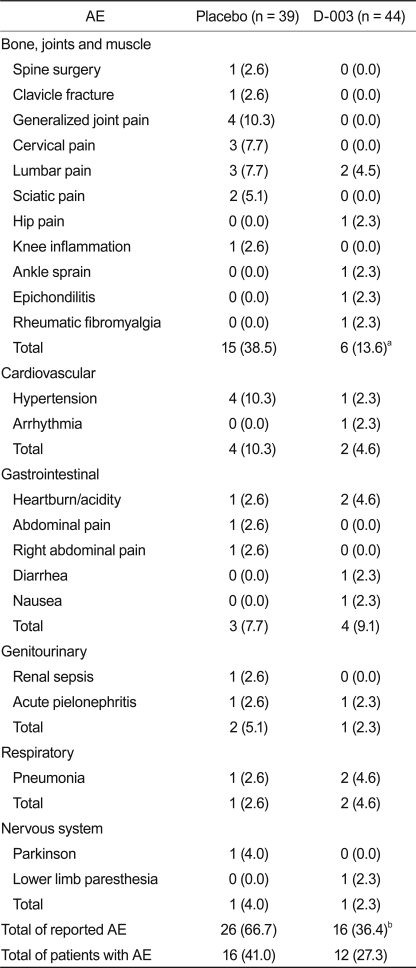
Overall, 28 subjects (16 placebo, 12 D-003) reported a total of 42 AEs during the study. The frequency of AE related to bone, joints, and muscle (15 from placebo, 6 from D-003) and the total frequency (26 from placebo, 16 from D-003) of AE in the D-003 group were lower than in the placebo group (p < 0.01 and p < 0.05 vs. placebo, respectively) (Table 4). Only one AE (spine surgery) was classified as serious, which occurred in a placebo-woman who discontinued the trial because of this reason.
This study demonstrated for the first time that D-003 (10 mg/day) administered to postmenopausal women with low BMD for 3 years significantly increased lumbar spine BMD (5.1% as compared to placebo); however, the effects of D-003 on femoral neck BMD did not reach statistical significance. As compared with the placebo, D-003 treatment improved pain levels, physical status, and quality of life, and reduced the rate of bone/joint/muscle complaints.
The study population had a number of osteoporosis risk factors: all were post-menopausal women and predominantly sedentary. Most of the patients had insufficient dietary intake of calcium, a family history of fractures, and were smokers. The women were, therefore, amenable to measures aimed at preventing osteoporosis development. These characteristics, efficacy variables, and concomitant therapies were well matched in both groups, which were homogeneous for all comparisons. It should be noted that the study women also displayed a great number of coronary risk factors, including post-menopausal status, hypercholesterolemia, smoking, hypertension arterial, obesity, and being overweight.
We previously demonstrated that D-003 (10 mg/day) treatment administered for 6 months to postmenopausal women with low BMD significantly reduced (33.7% as compared with placebo) the urinary excretion of DPD/Cr [29], a marker of bone resorption. These results support an effect comparable to that reported for bisphosphonates [34].
We have now found that 3 years of D-003 treatment also progressively increased lumbar spine BMD in postmenopausal women with BMD T scores below -1.0, while such values significantly decreased in the placebo group. It should be noted that this study did not include a mandatory intake of calcium supplements, which could explain why the progressive decline in BMD of patients in the placebo group was greater than in other studies, where calcium supplementation has been shown to retard bone loss [35].
Osteoporosis develops when bone resorption surpasses bone formation and bone turnover is accelerated. This creates a negative balance of bone remodeling as observed in post-menopause [1]. Thus, partial suppression of bone resorption rates constitutes the main strategy for managing osteoporosis, given that it should slow the rate of bone loss in patients with a negative bone remodeling balance. In addition, it should produce a mild increase in bone mass as formation catches up with resorption [4]. If the suppression of bone resorption is greater than bone formation at individual remodeling sites, beneficial effects on the focal remodeling balance are expected. Controlling excessive bone resorption with antiresorptive agents would reduce the number of potential sites for structural failure and would help to lower fracture rates [36], thus improving skeletal mass and strength.
The increase in lumbar spine BMD over the 3 years of treatment with D-003 10 mg/day is consistent with results reported for bisphosphonates [8-10,37-40]. The increase in dorsal spine BMD reported here (5.1% as compared with the placebo) was comparable to that achieved with alendronate 5 mg/day (5.67% vs. placebo) administered for 2 years to older women [37] and was greater than that reported for alendronate 10 mg/day given for 1 year to post-menopausal women receiving a calcium supplement (500 mg/day) (4.9% vs. placebo) [38] and in a comparative study of alendronate vs. raloxifene (2.4%) [39]. The increase in lumbar spine BMD with D-003 10 mg/day observed here, however, was 1.5% lower than that achieved (6.6% vs. placebo) with alendronate treatment administered for 4 years (5 mg/day for 2 years, 10 mg/day for the next 2 years) in postmenopausal women taking calcium supplementation [40].
Taken together these results support the hypothesis that the increase in lumbar spine BMD achieved after 3 years of D-003 treatment should be clinically meaningful. Specifically, the study subjects did not receive a daily calcium supplement, which together with the lower sample size of this trial, could have prevented us from obtaining significant differences between the effects of D-003 and placebo until after 3 years of therapy, as compared with the significant increases seen after 1 year on alendronate administered in conjunction with calcium supplementation [37,40].
While D-003 significantly increased BMD at the lumbar spine, the increase in femoral neck BMD did not reach statistical significance. This result is not surprising, since sites with large proportions of cancellous bone usually have higher turnover rates than predominately cortical sites. Therefore, the effects of bisphophonates on femoral neck BMD are lower, as compared to their effects on lumbar BMD [37,38,40].
The D-003-induced increase in BMD observed here demonstrated that BMD responds to a decrease in the remodeling space. D-003 therapy reduces increased bone resorption and does not affect bone formation, which may lead to a state in which bone formation exceeds bone resorption. This would allow for filling of previously initiated resorption cavities. Such transient remodeling effects alone, however, cannot explain the progressive increase in BMD over 3 years [36]. Nevertheless, despite the observation that D-003 does not increase bone formation, it reduced the apoptosis of osteoblasts [25,26], which, theoretically, could contribute to the longer action of these cells during cavity remodeling.
The effects of D-003 on BMD shown here are consistent with experimental data. D-003 oral treatment has been shown to reduce bone loss and bone resorption in ovx rats by increasing osteoclast apoptosis, without affecting bone formation [25-27]. The antiresorptive effects of D-003 are consistent with its inhibitory effects on mevalonate formation [20]. In addition, considering the link between increased lipid oxidation and osteoporosis [14], it is reasonable to hypothesize that the antioxidant effects of D-003 [22,24] should also contribute to its bone protective effects.
Based on the questionnaire, D-003 treatment also improved osteoporosis-related quality of life, including pain, physical status, and the total quality of life score, as compared with baseline and placebo. After 2 and 3 years of treatment, leisure activities decreased significantly in the placebo group, but not in D-003-treated patients. In consequence, leisure activity scores of this dimension were lower in the placebo group, as compared with the D-003-treated group. Since the ability to perform leisure activities can be influenced by pain perception and physical status, this result is consistent with the effects of D-003 on such domains and could depend, at least partially, on its bone protective effects. In addition, these results are consistent with the significant reduction in AE with regard to bones, joints, and muscles in D-003 patients, as compared with the placebo group. Nevertheless, we cannot discount the other effects of D-003, including those exerted on the vascular system, which could had contributed to a perceived improvement in quality of life in some study subjects.
Consistent with previous reports [21-23,29] D-003 significantly reduced serum LDL-C and TC, and increased HDL-C values, which should be beneficial for post-menopausal women with multiple lipid and non-lipid coronary risk factors.
The tolerability of D-003 was very good. Withdrawal rates due to AE were similar in the D-003 and placebo groups, whereas the rates of AE and AE associated with bones, joints, and muscles in the D-003 group were lower than in placebo patients. It is interesting, however, that the rate of fractures recorded in the trial was actually very low (only one placebo subject), which could be a result of two factors. First, the study only recorded explicit clinical fractures that were later confirmed radiologically, so that subclinical fractures, which frequently occur with vertebral fractures, were not detected. Secondly, most randomized women were relatively young and were either near or in the early stages of postmenopause.
Overall, this study has several limitations. First, while a reduction in fracture risk is the main goal of intervention in subjects with osteoporosis, this trial did not demonstrate an effect of D-003 on fracture risk, since a much larger sample size would be required to have adequate power to reliably detect a reduction in fractures. Second, this trial did not include information on bone strength or histological data and we cannot determine whether continuous daily treatment with D-003 impairs the rate of osteoid mineralization or individual osteoblast function. Third, we did not assess biochemical markers of mineral homeostasis (calcium, phosphate, and parathyroid hormone); therefore, we cannot evaluate the effect on such variables. Finally, the average age of participants at baseline was 55 years; thus, the results might not apply to older women.
When considering both the results and limitations, however, this study demonstrates a protective effect of D-003 based on the increase of lumbar BMD in postmenopausal women with low BMD. This finding is consistent with previous experimental and clinical data [25-29]. In addition, these results demonstrate the benefits of this treatment on osteoporosis-related quality of life indicators as well as on bone, joint, and muscle symptoms, as compared with the placebo.
Over the 3 years of the study, D-003 (10 mg/day) produced significant increases in lumbar spine BMD, but not femoral neck BMD, improved osteoporosis-related quality of life domains, and reduced adverse experiences related to bone, joints, and muscle, as compared with the placebo. Collectively, these findings suggest that D-003-treatment could be useful for managing post-menopausal women with spine T scores of -1.0 or more. D-003 also produced beneficial changes in lipid profiles that could be an additional benefit for this population. Lastly, D-003 was well tolerated. Additional larger studies, however, should confirm the present results and investigate the effects of D-003 on fracture risk before decisively concluding that there are potential benefits of D-003-treatment in the prevention and treatment of post-menopausal osteoporosis.
Acknowledgments
This study was supported by a research grant from the West Havana Scientific Pole. The personnel involved in this study did not receive any special financial compensation or preferential treatment as a result of their participation in the trial.
References
2. Wildner M, Peters A, Raghuvanshi VS, Hohnloser J, Siebert U. Superiority of age and weight as variables in predicting osteoporosis in postmenopausal white women. Osteoporos Int 2003;14:950–956PMID : 13680102.


3. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337–342PMID : 12748652.


4. Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol 2006;6:307–312PMID : 16650801.


5. van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun 1999;264:108–111PMID : 10527849.


6. van beek E, Lowik C, van der Pluijm G, Papapoulos S. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: a clue to the mechanism of action of nitrogen-containing bisphosphonates. J Bone Miner Res 1999;14:722–729PMID : 10320520.


7. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 2002;112:281–289PMID : 11893367.


8. Bone HG, Hosking D, Devogelaer JP, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004;350:1189–1199PMID : 15028823.


9. Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 2000;85:4118–4124PMID : 11095442.


10. Roux C, Seeman E, Eastell R, et al. Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin 2004;20:433–439PMID : 15119979.


11. Chesnut III CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004;19:1241–1249PMID : 15231010.


12. Boonen S, Laan RF, Barton IP, Watts NB. Effect of osteoporosis treatments on risk of non-vertebral fractures: review and meta-analysis of intention-to-treat studies. Osteoporos Int 2005;16:1291–1298PMID : 15986101.


13. Cranney A, Wells G, Willan A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev 2002;23:508–516PMID : 12202465.


14. Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol 2000;20:2346–2348PMID : 11073836.


15. Bauer DC. HMG CoA reductase inhibitors and the skeleton: a comprehensive review. Osteoporos Int 2003;14:273–282PMID : 12736772.


16. Bauer DC, Mundy GR, Jamal SA, et al. Use of statins and fracture: results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med 2004;164:146–152PMID : 14744837.


17. Rejnmark L, Buus NH, Vestergaard P, et al. Effects of simvastatin on bone turnover and BMD: a 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res 2004;19:737–744PMID : 15068496.


18. Reid IR, Hague W, Emberson J, et al. Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised controlled trial. Long-term Intervention with Pravastatin in Ischaemic Disease. Lancet 2001;357:509–512PMID : 11229669.


19. Mas R. D-003: antiplatelet therapy, treatment of lipoprotein disorders. Drugs Future 2004;29:773–786.

20. Menendez R, Mas R, Amor AM, Rodeiros I, Gonzalez RM, Alfonso JL. Inhibition of cholesterol biosynthesis in cultured fibroblasts by D003, a mixture of very long chain saturated fatty acids. Pharmacol Res 2001;44:299–304PMID : 11592864.


21. Menendez R, Mas R, Perez J, Gonzalez RM, Jimenez S. Oral administration of D-003, a mixture of very long chain fatty acids prevents casein-induced endogenous hypercholesterolemia in rabbits. Can J Physiol Pharmacol 2004;82:22–29PMID : 15052302.


22. Castano G, Menendez R, Mas R, et al. Effects of D-003, a new hypocholesterolaemic and antiplatelet compound, on lipid profile and lipid peroxidation in healthy volunteers. Clin Drug Investig 2003;23:193–203.


23. Castano G, Mas R, Fernandez L, et al. Effects of D-003 on the lipid profile of patients with type II hypercholesterolaemia: a phase II clinical study. Clin Drug Investig 2003;23:789–802.


24. Menendez R, Mas R, Amor AM, et al. Inhibition of rat lipoprotein lipid peroxidation by the oral administration of D003, a mixture of very long-chain saturated fatty acids. Can J Physiol Pharmacol 2002;80:13–21PMID : 11911221.


25. Mendoza S, Noa M, Mas R, Mendoza N. Effects of D-003 (5-200 mg/kg), a mixture of high molecular weight aliphatic acids from sugarcane wax, on bones and bone cell apoptosis in ovariectomized rats. Int J Tissue React 2005;27:213–222PMID : 16440587.

26. Mendoza S, Noa M, Mas R, Mendoza N. Comparison of the effects of D-003, a mixture of high-molecular-weight aliphatic acids from sugarcane wax, and pravastatin on bones and osteoclast apoptosis of ovariectomized rats. Drugs Exp Clin Res 2005;31:181–191PMID : 16425974.

27. Noa M, Mendoza S, Mas R, Mendoza N, Goicochea E. Long-term effects of D-003, a mixture of high molecular weight acids from sugarcane wax, on bones of ovariectomized rats: a one year study. Pharmazie 2008;63:486–488PMID : 18604996.

28. Noa M, Mendoza S, Mas R, Mendoza N, Leon F. Effect of D-003, a mixture of very high molecular weight aliphatic acids, on prednisolone-induced osteoporosis in Sprague-Dawley rats. Drugs R D 2004;5:281–290PMID : 15357627.


29. Ceballos A, Mas R, Castano G, et al. The effect of D-003 (10 mg/day) on biochemical parameters of bone remodelling in postmenopausal women: a randomized, double-blind study. Int J Clin Pharmacol Res 2005;25:175–186PMID : 16402634.

30. van Schoor NM, Knol DL, Glas CA, et al. Development of the Qualeffo-31, an osteoporosis-specific quality-of-life questionnaire. Osteoporos Int 2006;17:543–551PMID : 16362146.


31. Seigler L, Wu WT. Separation of serum high-density lipoprotein for cholesterol determination: ultracentrifugation vs precipitation with sodium phosphotungstate and magnesium chloride. Clin Chem 1981;27:838–841PMID : 7237761.


32. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502PMID : 4337382.


33. O'Brien PC, Shampo MA. Statistical considerations for performing multiple tests in a single experiment. 5. Comparing two therapies with respect to several endpoints. Mayo Clin Proc 1988;63:1140–1143PMID : 3193822.


34. Yang TS, Tsan SH, Chen CR, Chang SP, Yuan CC. Effects of alendronate on bone turnover markers in early postmenopausal women. Zhonghua Yi Xue Za Zhi (Taipei) 1998;61:568–576PMID : 9830233.

35. Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Long-term effects of calcium supplementation on bone loss and fractures in postmenopausal women: a randomized controlled trial. Am J Med 1995;98:331–335PMID : 7709944.


36. Heaney RP. The bone-remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res 1994;9:1515–1523PMID : 7817796.


37. Bone HG, Downs RW Jr, Tucci JR, et al. Dose-response relationships for alendronate treatment in osteoporotic elderly women: Alendronate Elderly Osteoporosis Study Centers. J Clin Endocrinol Metab 1997;82:265–274PMID : 8989272.


38. Pols HA, Felsenberg D, Hanley DA, et al. Fosamax International Trial Study Group. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Osteoporos Int 1999;9:461–468PMID : 10550467.


Figure 1
Changes in bone mineral density during 3 years of therapy with D-003 or placebo. ap < 0.01, comparison with placebo (Mann Whitney U test).
-
METRICS

-
- 0 Crossref
- 8 Scopus
- 12,031 View
- 122 Download
- Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


