 |
 |
| Korean J Intern Med > Volume 30(6); 2015 > Article |
|
Abstract
The incidence rate of gastric cancer in Korean Americans is over five times higher than that in non-Hispanic whites, and is similar to the incidence of colorectal cancer in the overall United States population. In Korea, the National Cancer Screening Program recommends endoscopy or upper gastrointestinal series for people aged 40 years and older every 2 years. However, the benefit of gastric cancer screening in Korean Americans has not been evaluated. Based on epidemiologic studies, Korean Americans appear to have more similar gastric cancer risk factors to Koreans as opposed to Americans of European descent, though the risk of gastric cancer appears to decrease for subsequent generations. Therefore, in accordance with recent recommendations regarding screening for gastric cancer in Korea, endoscopic screening for gastric cancer in Korean Americans should be considered, especially in those with known atrophic gastritis/intestinal metaplasia or a family history of gastric cancer. In the future, additional studies will needed to assess whether a screening program for gastric cancer in Korean Americans will result in a survival benefit.
The incidence of gastric cancer is estimated to be roughly 952,000 cases yearly, making it the fifth most common malignancy in the world, after lung, breast, colorectal and prostate [1,2]. More than 70% of cases occur in developing countries and half of all the worldwide cases occur in Eastern Asia [1]. The incidence of gastric cancer in the United States is low, especially in non-Hispanic White Americans (4.0 per 100,000 individuals) compared to Koreans (42.5 per 100,000 individuals) [3,4]. Furthermore, gastric cancer is the third leading cause of cancer death in both sexes worldwide. The highest estimated mortality rates are in Eastern Asia (24 per 100,000 in men and 9.8 per 100,000 in women), and the lowest in North America (2.8 per 100,000 in men and 1.5 per 100,000 in women) [1,2]. Asian Americans were the fastest growing immigrant group in the United States between 2000 and 2010 [5] and in 2011 totaled 18.2 million, with Korean Americans totaling 1.7 million.
Countries with high prevalences of gastric cancer have initiated screening programs, as early detection is associated with better outcomes [6]. In Korea, screening was initiated in 1999 [7] and involves upper endoscopy or upper gastrointestinal series (UGI) for patients 40 years or older every 2 years. As a result of this screening program, more than 50% of gastric cancers in Korea are diagnosed at an early stage [8], compared to fewer than 10% in Western countries [9]. Whether gastric cancer screening is beneficial in Koreans who have immigrated to the Unites States, and what those screening recommendations should be, remains unclear. According to a recent study surveying Koreans living in Washington State, roughly one-third of the responders had visited Korea for medical services within previous 5 years [10], further complicating potential screening recommendations. In this review article, we will address the potential benefits of gastric cancer screening and suggest a screening and surveillance strategy for Korean Americans.
There is significant variability in the incidence of gastric cancer among different ethnicities in the United States. According to SEER (Surveillance, Epidemiology and End Results) data from 2002 to 2006, Asian Americans had the highest incidence rates, followed by African Americans and Hispanics (Asian men, 20.8 per 100,000/year; African men, 18.4 per 100,000/year; and Hispanic men, 17.1 per 100,000/year) (Fig. 1) [11]. Korean Americans have a significantly higher incidence rate (50.0 per 100,000 men and 26.3 per 100,000 women) and a higher mortality rate (31.5 per 100,000 men and 14.5 per 100,000 women) compared to other East-Asians (Japanese and Chinese) and Southeast Asians (e.g., Vietnamese) (Fig. 2) [12]. Furthermore, a recent study of cancer incidence and mortality in East-Asian Americans in California found that Chinese, Vietnamese, Korean, and Japanese Americans had higher rates of gastric cancer and death from gastric cancer compared to non-Hispanic European Americans, with Koreans having the greatest risk among these groups [13,14].
In addition, first generation immigrants have a higher incidence of gastric cancer between first generation and subsequent generations of Asian Americans. According to the effect of migration on gastric cancer incidence among Japanese in Hawaii, first generation Japanese Americans still had very high rates of gastric cancer [15]. However, rates declined and became similar to those of Americans of European descent after two generations. This observation suggests that environmental factors have a significant influence on the development of gastric cancer. However, this situation may not be directly translatable to the situation for Korean Americans since incidence rates appear to remain high for subsequent generations of Korean Americans. Therefore, there may still be a need for gastric cancer screening beyond the first generation of Korean Americans, especially those having additional risk factors for gastric cancer (discussed in the next section).
Gastric cancer is a multifactorial disease with both host and environmental factors playing a role in its etiology. Identified risk factors for gastric cancer include Helicobacter pylori infection, smoking, alcohol, obesity, salt intake, presence of atrophic gastritis (AG) and intestinal metaplasia (IM), and family history of gastric cancer. There are only limited available data on the status of gastric cancer risk factors in Korean Americans [16]. However, Korean Americans appear to have risk factors that are more similar to Koreans rather than Americans, which might explain the above-mentioned high gastric cancer prevalence in Korean Americans [17]. The relative risks (RRs) of gastric cancer with each risk factor are summarized in Table 1, and each risk factor will be discussed in the following sections.
H. pylori infection triggers a series of inflammatory reactions, which can subsequently lead to chronic gastritis. Progression from chronic gastritis to gastric atrophy and IM is an early step of mucosal changes in the stomach that can then lead to dysplasia and cancer [18]. H. pylori has been classified as group 1 carcinogen by the International Agency for Research on Cancer since 1994 because many studies have proven the association between H. pylori infection and development of gastric cancer, especially intestinal type non-cardiac gastric cancer [19]. A pooled analysis of 12 case control studies including 762 cases of non-cardiac gastric cancer showed that the RR of H. pylori on gastric cancer was 2.97 (95% confident interval [CI], 2.34 to 3.77) [20].
H. pylori organisms can be found in the stomach in about two-thirds of the world’s population, and H. pylori infection is more prevalent in countries with high gastric cancer incidence rates. There is wide discrepancy in the prevalence of H. pylori between Koreans and Americans. In Korea, the prevalence of H. pylori in adults was about 66.9% in 1998, which decreased to 59.6% in 2005 [21]. In the United States, H. pylori prevalence is variable among ethnicities (60% in Hispanics, 54% in African American, and 20% in Whites) [22]. The estimated overall prevalence is about 20% for people < 30 years and 50% for those > 60 years in the United States. Although a well-designed study for the prevalence of H. pylori in Korean Americans has not been published yet, Korea-born Korean Americans are thought to have a higher prevalence of H. pylori infection than United States-born Korean Americans.
Smoking appears to be a moderate risk factor, compared with other tobacco-related cancers. A meta-analysis including 14,442 cases and 73,918 controls showed an odds ratio (OR) of 1.69 (95% CI, 1.35 to 2.11) for current smokers in comparison to those who have never smoked [23]. The risk for gastric cancer increases significantly with increasing amount and duration of smoking [24]. The effects of smoking may differ according to the location of gastric cancer; the RRs were 1.87 (95% CI, 1.31 to 2.67) for cardia cancers and 1.60 (95% CI, 1.41 to 1.80) for non-cardiac cancers in a meta-analysis based on nine cohort studies [25].
Drinking alcohol has been long suggested as a risk factor for gastric cancer. In a recent meta-analysis of 44 case control and 15 cohort studies including 34,500 cases of gastric cancer, the influence of light to moderate alcohol consumption results in a slight increase in risk, whereas heavy alcohol use (> 4 drinks per day) yields a more significant risk of 1.20 (95% CI, 1.01 to 1.44) [26]. However, in most prospective studies, the RR was not significantly elevated [27]. In a few studies that analyzed the risk for gastric cancer according to the tumor location, a slightly stronger association was found for non-cardia cancer than for cardia gastric cancer [26].
The daily sodium intake of Koreans is more than that of Americans (4,791 mg vs. 3,436 mg) [28]. The amount of daily salt intake is strongly related with the type of food consumed. Korean-Americans have a tendency to consume traditional Korean food (which has a high salt content, e.g., soy sauce, salt-preserved fish, and Korean style soup, etc.) even after immigrating to the United States. High dietary salt intake is known to be a risk factor for gastric cancer [29]. A meta-analysis including 11 studies (seven case controls and four cohorts) showed significant positive association between high salt intake and gastric cancer compared with low salt intake (OR, 2.05; 95% CI, 1.60 to 2.62) [30]. In another meta-analysis including only prospective population studies, high and moderately high salt intake were associated with increased risk of gastric cancer (RR, 1.68; 95% CI, 1.17 to 2.41; and RR, 1.41; 95% CI, 1.03 to 1.93, respectively) [31].
Unlike esophageal adenocarcinomas in which obesity is a major risk factor, studies on the association between obesity and gastric cancer have shown conflicting results. A recent meta-analysis including 24 prospective studies found that obesity (body mass index [BMI] ≥ 30 kg/m2) was not associated with risk of gastric cancer (RR, 1.06; 95% CI, 0.99 to 1.12) [32]. In a pooled analysis of esophagogastric junction cancers, individuals with BMI of 25 to 30 kg/m2 had a 1.21-fold (95% CI, 1.03 to 1.42), and those with BMI of ≥ 30 kg/m2 had a 2.06-fold (95% CI, 1.63 to 2.61) increased risk of esophagogastric junctional cancer, including cardia gastric cancers, compared to individuals with BMI of < 25 kg/m2 [33]. In contrast, obesity is not a risk factor for non-cardia gastric cancer [34].
AG and IM have been regarded as pre-cancerous conditions of gastric cancer, and have a strong relation with H. pylori infection [35]. Therefore, AG and IM have been suggested as a useful clinical entity to identify those who are at higher risk for gastric cancer who would benefit from surveillance endoscopy. A nationwide cohort study in Netherlands reported that risk of gastric cancer increased in a step-wise manner correlating to the severity of premalignant gastric lesions (annual incidence of gastric cancer within 5 years after diagnosis: 0.1%, 0.25%, 0.6%, and 6% for AG, IM, mild-to-moderate dysplasia, and severe dysplasia, respectively) [36]. Although the risk of gastric cancer in individuals with AG differs according to the severity of AG, the adjusted RR of gastric cancer in the patients having severe AG was 5.76 [37].
IM is generally sub-classified as complete and incomplete type. Complete type (small intestinal type) IM expresses the full set of digestive enzymes, while incomplete type (colonic type) IM shows absent or incomplete expression. Incomplete type is considered to be the most advanced stage of IM and has higher risk of progressing on to gastric cancer [38]. A pooled analysis including 14 cross-sectional studies and 10 follow-up studies revealed that 13 out of 14 cross-sectional studies and 6 of 10 follow-up studies reported statistically significant higher gastric cancer prevalence in incomplete type IM than in complete type IM, and the RR of gastric cancer ranged from 4 to 11 [39]. Therefore, patients that have incomplete type IM should be considered for gastric cancer surveillance. Furthermore, H. pylori-infected individuals with IM were found to have a 6.5-fold higher risk of developing gastric cancer [40].
Members of the same family tend to have shared genetic factors as well as similar environmental factors such as socioeconomic status and dietary habit. Therefore, caution should be used when interpreting the results of studies that report family history of gastric cancer as an independent risk factor of gastric cancer. A Korean study showed that adjusted OR for gastric cancer was 2.85 (95% CI, 1.83 to 4.46) for subjects with first-degree relatives with gastric cancer [41]. In addition, in a recent meta-analysis evaluating H. pylori infection and gastric histology in first-degree relatives of gastric cancer patients, family history of gastric cancer increases the risk of H. pylori infection, AG, and IM by approximately 2-fold each [42].
Four methods have been used in gastric cancer screening: H. pylori serology, serum pepsinogen (PG) testing, UGI series, and endoscopy. The ideal screening test should be simple, safe, validated and cost-effective. Pros and cons of each screening method for gastric cancer are summarized in Table 2.
H. pylori serology itself is not an effective method of screening for gastric cancer because of low sensitivity and inability to detect premalignant lesions. In fact, it can be negative in the presence of longstanding AG and IM. Even though H. pylori virulence factors such as Cag A, Vac A, and Bab A may increase the sensitivity for detecting premalignant lesions, their sensitivity remains low [43]. Therefore, H. pylori serology alone is not useful as a screening test for gastric cancer.
PG is a precursor of pepsin produced in the gastric mucosa. Biochemically and immunologically, PG is classified into two different isozymes, namely PG I and PG II. PG I is produced by chief cells in the fundus and body, and PG II is produced throughout the entire stomach [44,45]. The serum PG level reflects not only the morphology and function of acid secretory glands but also pathological conditions of the gastric mucosa such as inflammation or atrophy [46-48]. A stepwise decrease in the serum PG I level and PG I/II ratio is closely associated with the progression of gastric atrophy [49,50]. Therefore, the serum PG level is considered to be a useful marker of AG, which is a precursor to intestinal type adenocarcinoma in the stomach.
To detect gastric cancer, the serum PG level, followed by endoscopic examination, was introduced in mass screening of high-risk patients for gastric cancer. In the case of PG I ≤ 70 ng/L and PG I/II ratio ≤ 3.0, the risk for gastric cancer increases. In fact, several prospective cohort studies reported that serum PG level is useful for evaluating the risk for gastric cancer [51-53]. A case-control study using serum PG level for gastric cancer screening showed the ORs for death from gastric cancer among control subjects screened within 1 and 2 years were 0.238 (95% CI, 0.061 to 0.929) and 0.375 (95% CI, 0.155 to 0.905), respectively [54]. In a pooled meta-analysis assessing approximately 300,000 people, the sensitivity and specificity of serum PG testing for gastric cancer screening were 77% and 73%, respectively [55]. Therefore, gastric cancer screening using serum PG level is suggested to decrease mortality from gastric cancer. However, it is unclear if this data is applicable to populations outside of Japan.
The barium meal indirect X-ray examination was introduced as a mass screening program of gastric cancer in the 1960s in Japan [56]. It is used widely in resident medical examinations under the National Cancer Screening for the aged, employer medical examinations, and personal medical examinations. Gastric cancer screening using UGI series suggests a reduction of mortality in a meta-analysis that included three Japanese case-control studies; however, the level of evidence is weak [57]. The sensitivity of UGI series ranged from 60% to 80%, whereas the specificity and true positive rates were 90% and 0.7% to 2.0%, respectively [48]. Most case-control studies conducted in Japan showed that screening by UGI resulted in a 40% to 60% reduction in gastric cancer mortality [58-60]. However, because of the lack of data from prospective series, that defined death from gastric cancer as an endpoint, UGI series receives a low grade recommendation for population-based gastric cancer screening [61].
Although UGI series was the initial tool for mass screening for gastric cancer, a cohort analysis showed that the detection rate of early gastric cancer by endoscopy was approximately about 3- to 5-fold higher than that by UGI series and use of endoscopy was more cost-effective [62]. The sensitivity of endoscopy for gastric cancer screening was 78% to 84% [63,64]. As a result, endoscopy has become the main modality for gastric cancer screening in Korea and Japan. Although it has been suggested that endoscopic screening is cost-effective in high-incidence areas, there is no evidence that endoscopic screening is effective or cost-effective in average-risk populations [65]. Population-based endoscopic screening for gastric cancer has several limitations such as the need for experienced endoscopists, potential complication of endoscopy, and low acceptability by participants [48]. In addition, endoscopy may not be a practical strategy for mass screening in other countries where the cost of endoscopy is higher.
In Korea, the National Cancer Screening Program for gastric cancer screening was initiated in 1999 [7]. Endoscopy or UGI series is recommended for people aged 40 years and older every 2 years. In 2001 and 2003, the participation rate was only 11.4% and 13.6%, respectively [66]. Because of the government’s efforts to encourage cancer screening through media and by offering free screening, the rate of participation has been increasing [67]. The participation rate for gastric cancer screening in 2012 was 43.9% [68]. With increasing participation in gastric cancer screening, the number of patients diagnosed with gastric cancer through this screening program has increased, and has resulted in a higher proportion of early-stage cancer being diagnosed. This has resulted in an increased use of minimally invasive treatments such as endoscopic submucosal dissection [8]. As a result, approximately 46% to 67% of gastric cancers detected with endoscopy were early-stage cancers [69], and the 5-year survival rate has increased from 42.8% in 1993 to 1995, 46.6% in 1996 to 2000, 57.7% in 2001 to 2005, to 69.4% in 2006 to 2011 (Fig. 3) [4].
In a study comparing the performance of endoscopy with that of UGI series for gastric cancer screening, gastric cancer detection rates for endoscopy and UGI series were 2.61 per 1,000 and 0.68 per 1,000 screenings, respectively [70]. The sensitivity of endoscopy and UGI series screening to detect gastric cancer was 69.0% and 36.7%, respectively, and the specificity was 96.0% and 96.1%, respectively [69,70]. Recently, a study evaluating the cost-effectiveness of the National Gastric Cancer Screening Program demonstrated that the age-adjusted incremental cost-effective ratio for survival in endoscopy was 119,099,000 to 178,700,000 Korean won/survival, which was lower than that in UGI series (260,201,000 to 371,011,000 won/survival) [71]. In addition, it was determined that it would costs approximately 14,466,000 to 15,014,000 won per life-year saved for UGI series and 8,817,000 to 9,755,000 won per life-year saved for endoscopy. Furthermore, studies comparing endoscopy versus no screening demonstrated that endoscopy was the most cost-effective strategy [71-73]. Therefore, endoscopy is recommended as the first-line screening method in Korea.
AG, IM, and dysplasia are considered precancerous lesions and require accurate surveillance programs. In a large western cohort study, the annual incidence of gastric cancer within 5 years was 0.1% for AG, 0.25% for IM, 0.6% for mild-to-moderate dysplasia, and 6% for severe dysplasia [36]. In another cohort study, the risk of progression to cancer within 10 years was 0.8% for AG, 1.8% for IM and 3.9% for low-grade dysplasia [74].
Currently, there are no international recommendations for surveillance of premalignant lesions. Recently, the International Consensus Project suggested the following recommendations for the management of patients with precancerous conditions [75]: (1) patients with mild-to-moderate atrophy and/or IM only in the antrum do not need follow-up (evidence level 4, recommendation grade D); (2) patients with extensive atrophy and/or extensive IM should be offered endoscopic surveillance (evidence level 2++, recommendation grade B) every 3 years (evidence level 4, recommendation grade D); (3) patients with low-grade dysplasia should be followed up every 12 months while those with high-grade dysplasia should be closely followed up every 6 months (evidence level 2+, recommendation grade C); and (4) patients with dysplasia or cancer within an endoscopically visible lesion should undergo staging and resection.
However, discrepancy between the results of endoscopic forceps biopsy and endoscopic resection is not uncommon [76,77], with approximately half of lesions interpreted as high-grade dysplasia on forceps biopsy being diagnosed as carcinoma after endoscopic resection [78]. Therefore, in cases with high-grade dysplasia, most clinicians agree that complete endoscopic resection or surgical removal of the tumor is necessary instead of close follow-up.
It remains unclear who should be screened, when screening should be initiated, and how screening should be performed. It has been suggested that the screening strategy for gastric cancer should be based on incidence of the population and individual risk. The strategy of test and treat for H. pylori infection can be effective at reducing the incidence and mortality of gastric cancer in communities with a high incidence of gastric cancer. However, follow-up surveillance for gastric cancer is also necessary in patients requiring H. pylori eradication, because the risk of gastric cancer remains even after eradication of H. pylori. Therefore, the strategy of test and treat for H. pylori infection followed by evaluation with endoscopy to identify those who have developed AG or IM is needed in order to identify patients who are at higher risk of developing gastric cancer that should go on for surveillance.
In the United States, where gastric cancer incidence rates are low, a study that compared endoscopy with no screening showed that one-time screening for the general population at the age of 50 would cost $115,664 per quality-adjusted life year [79]. Furthermore, endoscopic screening of less advanced lesions was not cost-effective, except possibly for immigrants from high-risk Asian countries [80]. According to a guideline on ethnic issues in endoscopy by the American Society for Gastrointestinal Endoscopy [81], although screening for and treating H. pylori has the potential to reduce the risk for gastric cancer in groups with high gastric cancer risk, ethnicity based deviations from usual care is not suggested. In patients with gastric IM, surveillance is suggested for those at increased risk of gastric cancer due to ethnic background or a family history. Therefore, in accordance with recent recommendations regarding screening for gastric cancer in populations within Korea and Japan [82], endoscopic screening for gastric cancer in new (first generation) United States immigrants from high-risk regions around the world, such as Korea, Japan, and China, should be considered, especially if there is a family history of gastric cancer in a first-degree relative [81,83].
Although the evidence that Korean Americans have a high risk of gastric cancer similar to Koreans is clear, detailed data regarding the incidence according to generational status is lacking. In addition, differences in healthcare resources, screening strategy costs, and other healthcare-related factors exist. These differences require further investigation through more systematic, large scale studies. We have proposed a modified screening and surveillance program according to the presence of H. pylori infection with or without AG/IM or family history of gastric cancer in Korean Americans (Fig. 4). We recommend that Korean Americans undergo a screening EGD with biopsies (to determine H. pylori and AG/IM status) at the age of 40. Those at very low risk for gastric cancer (no H. pylori, no AG/IM) we suggest no further follow-up. Those at low risk (H. pylori infected, no AG/IM) should have H. pylori eradication therapy, and then undergo endoscopic examination every 3 to 5 years. In addition, H. pylori eradication should be confirmed. Those at high risk (presence of AG/IM, or family history of gastric cancer) should have endoscopy every 1 to 2 years with eradication therapy in cases with H. pylori infection. Those having low-grade dysplasia should have follow-up endoscopy in 6 to 12 months followed by annually endoscopic examination or undergo endoscopic resection if there is a visible lesion. Those having high-grade dysplasia should undergo endoscopic resection or follow-up endoscopy every 3 months. In the future, additional studies are needed to assess whether this screening program for gastric cancer in Korean Americans results in a survival benefit.
Based on epidemiologic studies, the incidence rate of gastric cancer in Korean Americans is over five times higher compared to non-Hispanic Whites. In addition, Korean Americans appear to have similar gastric cancer risk factors in terms of environmental exposures and genetic background as Koreans; although the risk of developing gastric cancer appears to decrease with subsequent generations. Therefore, in accordance with recent recommendations regarding screening for gastric cancer in Korea, endoscopic screening for gastric cancer in Korean Americans should be considered, especially in those with AG/IM or a family history of gastric cancer. However, to establish a systematic gastric cancer screening program in Korean Americans, there are several challenges such as identifying who should undergo screening, at what age should screening be initiated, and how should screening be performed. We have proposed an algorithm for gastric cancer screening in Korean Americans primarily based on data from the experience in Korea. In the future, prospective studies in a large population of Korean Americans will be needed to determine if gastric cancer screening is effective.
Figure 1.
Gastric cancer incidence rates according to ethnic groups in the United States (1992 to 2009). Adapted from Lui et al., with permission from Springer [11].
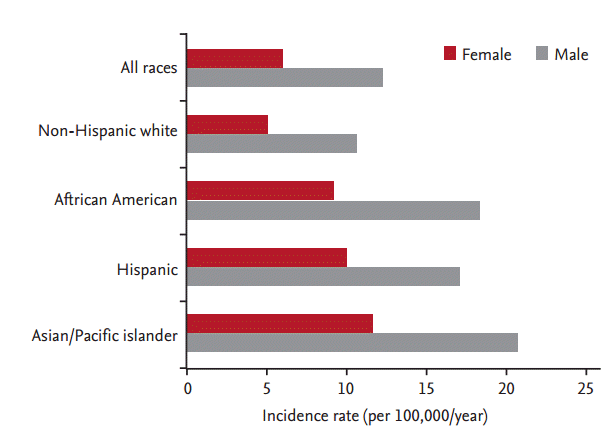
Figure 2.
Age-adjusted gastric cancer incidence (A) and mortality (B) rates in Asian Americans (1998 to 2002). Adapted from Miller et al., with permission from Springer [12].
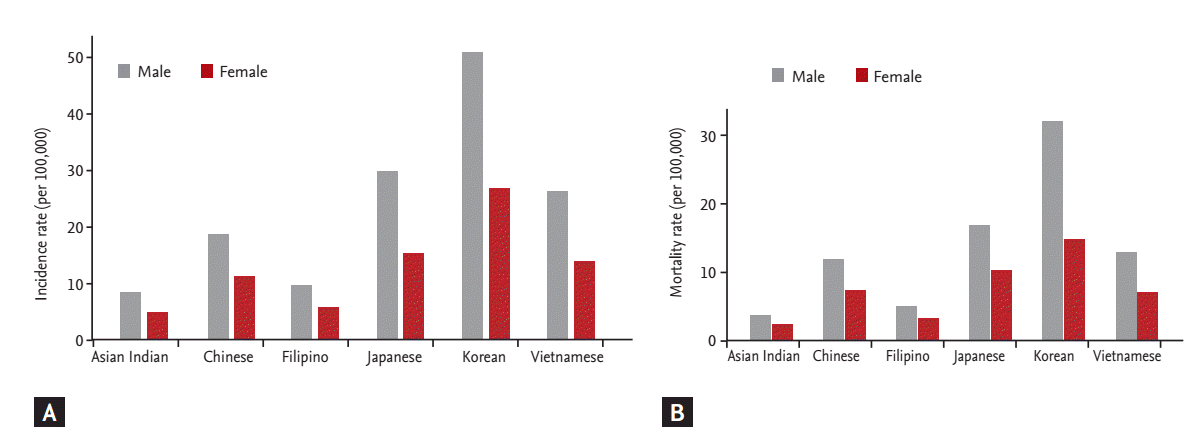
Figure 3.
Trends in the 5-year survival rates (%) of gastric cancer by year of diagnosis in Korea (1993 to 2011). Adapted from Jung et al. [4].
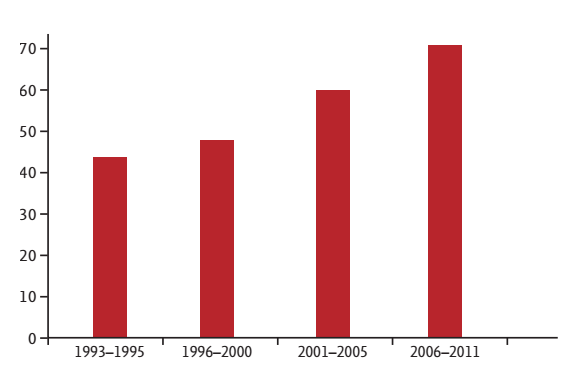
Figure 4.
Suggested screening and surveillance program for gastric cancer in Korean American. EGD, esophagogastroduodenoscopy; HP, Helicobacter pylori; AG, atrophic gastritis; IM, intestinal metaplasia; F/Hx, family history of gastric cancer; LGD, low-grade dysplasia; HGD, high-grade dysplasia; F/U, follow-up; ER, endoscopic resection.
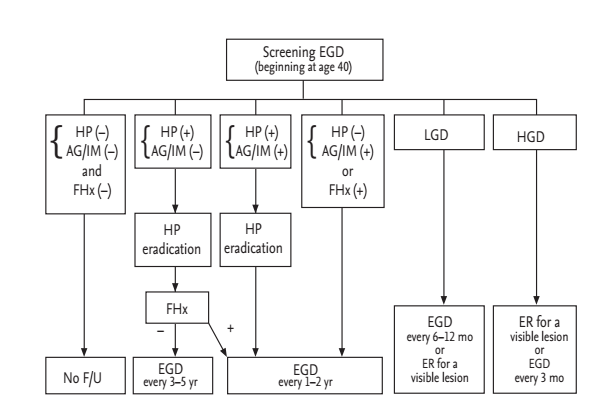
Table 1.
Summary of risk factors for gastric cancer
| Study | Factor | Risk estimate (95% CI) |
|---|---|---|
| Helicobacter and Cancer Collaborative Group (2001) [20] | H. pylori infection | RR, 2.97 (2.34–3.77) |
| La Torre et al. (2009) [23] | Cigarette smoking | OR, 1.69 (1.35–2.11) |
| Tramacere et al. (2012) [26] | Alcohol drinking | RR, 1.20 (1.01–1.44) |
| Ge et al. (2012) [30] | Salt intake | OR, 2.05 (1.60–2.62) |
| Chen et al. (2013) [32] | Obesity | RR, 1.06 (0.99–1.12) |
| Shin et al. (2010) [41] | Family history of gastric cancer | OR, 2.85 (1.83–4.46) |
Table 2.
Pros and cons of each screening method for gastric cancer
REFERENCES
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386.


2. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet] Lyon (FR): c2015. International Agency for Research on Cancer, [cited 2015 Aug 24]. Available from: http://globocan.iarc.fr.
3. Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723–1728.



4. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat 2014;46:109–123.




5. Hoeffel EM, Rastogi S, Kim MO, Shahid H. The Asian population: 2010 [Internet] Washington (DC): U.S: Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau, 2012. [cited 2015 Aug 24]. Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf.
6. Kong SH, Park DJ, Lee HJ, et al. Clinicopathologic features of asymptomatic gastric adenocarcinoma patients in Korea. Jpn J Clin Oncol 2004;34:1–7.


8. Kim YG, Kong SH, Oh SY, et al. Effects of screening on gastric cancer management: comparative analysis of the results in 2006 and in 2011. J Gastric Cancer 2014;14:129–134.



10. Oh KM, Jun J, Zhou Q, Kreps G. Korean American women’s perceptions about physical examinations and cancer screening services offered in Korea: the influences of medical tourism on Korean Americans. J Community Health 2014;39:221–229.


11. Lui FH, Tuan B, Swenson SL, Wong RJ. Ethnic disparities in gastric cancer incidence and survival in the USA: an updated analysis of 1992-2009 SEER data. Dig Dis Sci 2014;59:3027–3034.


12. Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control 2008;19:227–256.



13. McCracken M, Olsen M, Chen MS Jr, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin 2007;57:190–205.


14. Gomez SL, Noone AM, Lichtensztajn DY, et al. Cancer incidence trends among Asian American populations in the United States, 1990-2008. J Natl Cancer Inst 2013;105:1096–1110.



15. Maskarinec G, Noh JJ. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis 2004;14:431–439.

16. Park SY, Murphy SP, Sharma S, Kolonel LN. Dietary intakes and health-related behaviours of Korean American women born in the USA and Korea: the Multiethnic Cohort Study. Public Health Nutr 2005;8:904–911.


17. Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control 2007;14:78–85.


19. Schistosomes, liver flukes and Helicobacter pylori: IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 1994;61:1–241.


20. Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001;49:347–353.



21. Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter 2007;12:333–340.


22. Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis 2000;181:1359–1363.


23. La Torre G, Chiaradia G, Gianfagna F, et al. Smoking status and gastric cancer risk: an updated meta-analysis of case-control studies published in the past ten years. Tumori 2009;95:13–22.


24. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (Lyon) Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. Lyon: IARC Press, 2004;(International Agency for Research on Cancer, ed. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; vol. 83).
25. Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008;19:689–701.


26. Tramacere I, Negri E, Pelucchi C, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol 2012;23:28–36.


27. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum 2010;96:3–1383.


28. Elliott P, Brown I. Sodium Intakes around the World. Geneva: World Health Organization, 2007.
29. Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci 2005;96:1–6.


30. Ge S, Feng X, Shen L, Wei Z, Zhu Q, Sun J. Association between habitual dietary salt intake and risk of gastric cancer: a systematic review of observational studies. Gastroenterol Res Pract 2012;2012:808120.




31. D’Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr 2012;31:489–498.


32. Chen Y, Liu L, Wang X, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev 2013;22:1395–1408.


33. Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol 2012;41:1706–1718.



34. Lin XJ, Wang CP, Liu XD, et al. Body mass index and risk of gastric cancer: a meta-analysis. Jpn J Clin Oncol 2014;44:783–791.


35. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–6740.

36. de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 2008;134:945–952.


37. Tatsuta M, Iishi H, Nakaizumi A, et al. Fundal atrophic gastritis as a risk factor for gastric cancer. Int J Cancer 1993;53:70–74.


38. Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol 2009;24:193–201.


39. Gonzalez CA, Sanz-Anquela JM, Gisbert JP, Correa P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk: a review of the evidence. Int J Cancer 2013;133:1023–1032.



40. Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–789.


41. Shin CM, Kim N, Yang HJ, et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol 2010;44:e34–e39.


42. Rokkas T, Sechopoulos P, Pistiolas D, Margantinis G, Koukoulis G. Helicobacter pylori infection and gastric histology in first-degree relatives of gastric cancer patients: a meta-analysis. Eur J Gastroenterol Hepatol 2010;22:1128–1133.


43. Ley C, Mohar A, Guarner J, et al. Screening markers for chronic atrophic gastritis in Chiapas, Mexico. Cancer Epidemiol Biomarkers Prev 2001;10:107–112.

44. Samloff IM. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology 1971;61:185–188.


45. Samloff IM, Liebman WM. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology 1973;65:36–42.


46. Samloff IM, Stemmermann GN, Heilbrun LK, Nomura A. Elevated serum pepsinogen I and II levels differ as risk factors for duodenal ulcer and gastric ulcer. Gastroenterology 1986;90:570–576.


47. Miki K, Ichinose M, Shimizu A, et al. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn 1987;22:133–141.


48. Kato M, Asaka M. Recent development of gastric cancer prevention. Jpn J Clin Oncol 2012;42:987–994.


49. Borch K, Axelsson CK, Halgreen H, et al. The ratio of pepsinogen A to pepsinogen C: a sensitive test for atrophic gastritis. Scand J Gastroenterol 1989;24:870–876.


50. Asaka M, Kato M, Kudo M, et al. Relationship between Helicobacter pylori infection, atrophic gastritis and gastric carcinoma in a Japanese population. Eur J Gastroenterol Hepatol 1995;7 Suppl 1:S7–S10.
51. Yanaoka K, Oka M, Mukoubayashi C, et al. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev 2008;17:838–845.


52. Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut 2005;54:764–768.



53. Oishi Y, Kiyohara Y, Kubo M, et al. The serum pepsinogen test as a predictor of gastric cancer: the Hisayama study. Am J Epidemiol 2006;163:629–637.


54. Yoshihara M, Hiyama T, Yoshida S, et al. Reduction in gastric cancer mortality by screening based on serum pepsinogen concentration: a case-control study. Scand J Gastroenterol 2007;42:760–764.


55. Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, et al. Validity of serum pepsinogen I/II ratio for the diagnosis of gastric epithelial dysplasia and intestinal metaplasia during the follow-up of patients at risk for intestinal-type gastric adenocarcinoma. Neoplasia 2004;6:449–456.



56. Hisamichi S, Sugawara N. Mass screening for gastric cancer by X-ray examination. Jpn J Clin Oncol 1984;14:211–223.

57. Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010;15:1–20.

58. Abe Y, Mitsushima T, Nagatani K, Ikuma H, Minamihara Y. Epidemiological evaluation of the protective effect for dying of stomach cancer by screening programme for stomach cancer with applying a method of case-control study: a study of a efficient screening programme for stomach cancer. Nihon Shokakibyo Gakkai Zasshi 1995;92:836–845.

59. Inaba S, Hirayama H, Nagata C, et al. Evaluation of a screening program on reduction of gastric cancer mortality in Japan: preliminary results from a cohort study. Prev Med 1999;29:102–106.


60. Fukao A, Tsubono Y, Tsuji I, Hisamichi S, Sugahara N, Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer 1995;60:45–48.


61. Compare D, Rocco A, Nardone G. Screening for and surveillance of gastric cancer. World J Gastroenterol 2014;20:13681–13691.



62. Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol 2006;12:4873–4874.


63. Hosokawa O, Hattori M, Takeda T, Watanabe K, Fujita M. Accuracy of endoscopy in detecting gastric cancer. J Gastroenterol Mass Surv 2004;42:33–39.
64. Otsuji M, Kouno Y, Otsuji A, Tokushige J, Shimotatara K, Arimura K. Assessment of small diameter panendoscopy for diagnosis of gastric cancer: comparative study with follow-up survey date. Stomach Intest 1989;24:1291–1297.
65. Choi KS, Kwak MS, Lee HY, Jun JK, Hahm MI, Park EC. Screening for gastric cancer in Korea: population-based preferences for endoscopy versus upper gastrointestinal series. Cancer Epidemiol Biomarkers Prev 2009;18:1390–1398.


66. Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011;12:725–730.

67. Jung M. National Cancer Screening Programs and evidence-based healthcare policy in South Korea. Health Policy 2015;119:26–32.


68. National Cancer Center. Cancer Facts & Figures 2014. Goyang (KR): National Cancer Center, 2014.
69. Choi KS, Jun JK, Lee HY, et al. Performance of gastric cancer screening by endoscopy testing through the National Cancer Screening Program of Korea. Cancer Sci 2011;102:1559–1564.


70. Choi KS, Jun JK, Park EC, et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One 2012;7:e50041.



71. Cho E, Kang MH, Choi KS, Suh M, Jun JK, Park EC. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev 2013;14:2533–2540.


72. Lee HY, Park EC, Jun JK, Choi KS, Hahm MI. Comparing upper gastrointestinal X-ray and endoscopy for gastric cancer diagnosis in Korea. World J Gastroenterol 2010;16:245–250.



73. Chang HS, Park EC, Chung W, et al. Comparing endoscopy and upper gastrointestinal X-ray for gastric cancer screening in South Korea: a cost-utility analysis. Asian Pac J Cancer Prev 2012;13:2721–2728.


74. Tava F, Luinetti O, Ghigna MR, et al. Type or extension of intestinal metaplasia and immature/atypical “indefinite-for-dysplasia” lesions as predictors of gastric neoplasia. Hum Pathol 2006;37:1489–1497.


75. Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012;44:74–94.



76. Szaloki T, Toth V, Nemeth I, Tiszlavicz L, Lonovics J, Czako L. Endoscopic mucosal resection: not only therapeutic, but a diagnostic procedure for sessile gastric polyps. J Gastroenterol Hepatol 2008;23:551–555.


77. Lee CK, Chung IK, Lee SH, et al. Is endoscopic forceps biopsy enough for a definitive diagnosis of gastric epithelial neoplasia? J Gastroenterol Hepatol 2010;25:1507–1513.


78. Lim H, Jung HY, Park YS, et al. Discrepancy between endoscopic forceps biopsy and endoscopic resection in gastric epithelial neoplasia. Surg Endosc 2014;28:1256–1262.


79. Gupta N, Bansal A, Wani SB, Gaddam S, Rastogi A, Sharma P, et al. Endoscopy for upper GI cancer screening in the general population: a cost-utility analysis. Gastrointest Endosc 2011;74:610–624e2.


80. Yeh JM, Hur C, Kuntz KM, Ezzati M, Goldie SJ. Cost-effectiveness of treatment and endoscopic surveillance of precancerous lesions to prevent gastric cancer. Cancer 2010;116:2941–2953.



81. Cash BD, Banerjee S, Anderson MA, et al. Ethnic issues in endoscopy. Gastrointest Endosc 2010;71:1108–1112.


-
METRICS

- Related articles
-
Roadmap for elimination of gastric cancer in Korea2015 March;30(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


