 |
 |
| Korean J Intern Med > Volume 36(2); 2021 > Article |
|
Abstract
Background/Aims
We investigated the concordance rate of the classification of polymyositis (PM) and dermatomyositis (DM) between the Bohan and Peter criteria and the 2017 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria for idiopathic inflammatory myopathies (IIMs) (the 2017 EULAR/ACR criteria) in Korean patients.
Methods
We retrospectively reviewed the medical records of 137 patients with PM and DM. We finally included 72 PM patients and 49 DM patients who fulfilled the Bohan and Peter criteria for PM and DM and reclassified them by the 2017 EULAR/ ACR criteria.
Results
Three patients (4.2%) with probable PM were newly reclassified as non-IIM due to a total score of 5.3 or smaller. Meanwhile, one patient with possible PM was newly reclassified as probable PM due to the presence of dysphagia. In addition, eight patients (16.3%) with possible DM with DM-specific typical skin rash were newly reclassified as amyopathic DM (ADM) due to the absence of proximal muscle weakness. The concordance rate of the classification between the Bohan and Peter criteria and the 2017 EULAR/ACR criteria was 95.8% for PM patients and 83.7% for DM patients.
Conclusions
The Bohan and Peter criteria were comparable to the 2017 EULAR/ ACR criteria for classifying PM and DM in Korean patients. Considering the convenience of the Bohan and Peter criteria in the real clinical settings, we suggest that the old criteria should be preferentially applied and then performing muscle biopsy should be considered in a patient suspected of PM without antihistidyl tRNA synthetase (anti-Jo-1). Moreover, we suggest that ADM could also clinically be classified by the old criteria.
Idiopathic inflammatory myopathies (IIMs) are a group of chronic autoimmune diseases which are characterised by muscle weakness and various related symptoms. IIM can be classified into several subgroups according to typical clinical manifestations and histological findings, such as polymyositis (PM), dermatomyositis (DM) and inclusion body myositis (IBM) [1]. PM often presents proximal muscle weakness, neck flexor muscle weakness and dysphagia [2]. Meanwhile, DM is classified based on DM-specific typical skin rashes such as heliotrope rash and GottronŌĆÖs papule or histological features with proximal muscle weakness. DM without muscle involvement is called amyopathic DM (ADM) [3]. DM occurs in both children and adults, whereas PM predominantly occurs in elder adults [4]. The incidence of IIM was reported to range from 5 to 7.4 cases per million populations and it has been increasing in recent years [5,6].
So far, several classification criteria for IIM have been suggested and the classification criteria for PM and DM proposed by Bohan and Peter in 1975 (the Bohan and Peter criteria) have widely been used among them [7,8]. The Bohan and Peter criteria consist of five items: (1) symmetrical weakness, usually progressive, of the limb-girdle muscles; (2) elevation of skeletal muscle enzyme levels; (3) abnormal electromyography results; (4) muscle biopsy abnormalities; (5) typical skin rash of DM. Definite PM can be classified when all first four items are met, whereas definite DM can be classified when DM-specific typical skin rash plus three of other items are met [7,8].
However, the Bohan and Peter criteria have several limitations such as a monocentric data based on clinical observation, no detailed mention of typical DM rashes, biopsy findings or myositis-specific autoantibodies. Moreover, they have no instruction for exclusion or sub-classification. It is difficult to differentiate IIM from other medical conditions including peripheral neurological diseases and muscular dystrophies, and in particular, they could not discriminate between PM and IBM [9,10]. To overcome these limitations, the 2017 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria for adult and juvenile idiopathic inflammatory myopathies (the 2017 EULAR/ACR criteria) were recently established. The 2017 EULAR/ACR criteria are based on the scoring system and a score of < 5.3 may rule out IIM (non-IIM) [11]. Nevertheless, the Bohan and Peter criteria are still being used for classifying IIM due to the small number of items and convenience in the real clinical settings. For this reason, we wondered whether we should apply the new criteria to all patients suspected of IIM instead of the Bohan and Peter criteria. Till now, there was no report on the concordance rate of the classification of PM and DM between the Bohan and Peter criteria and the 2017 EULAR/ACR criteria in Korean patients. Hence, in this study, we applied the 2017 EULAR/ACR criteria to Korean patients with PM and DM, who were classified by the Bohan and Peter criteria at diagnosis, and investigated the concordance rate of the classification between the two criteria.
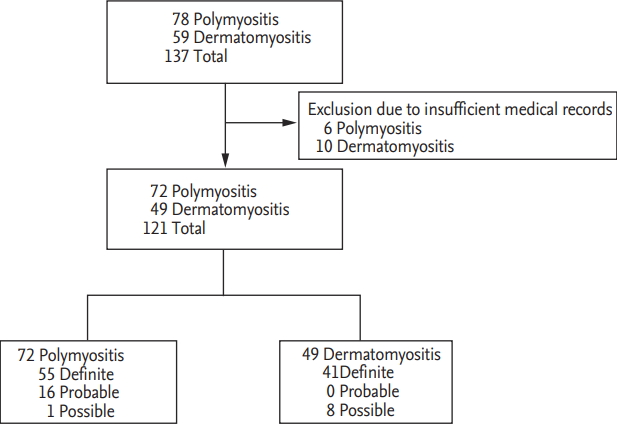
We retrospectively reviewed the medical records of 137 patients with IIM, which were identified in the 10th revised International Classification Diseases (ICD-10), and included 121 patients with IIM (72 PM patients and 49 DM patients) in this study. Sixteen patients with IIM were excluded due to insufficient medical records. Among 72 PM patients, 55 patients were classified as definite PM, 16 patients as probable PM and one patient as possible PM. Among 49 DM patients, 41 DM patients were diagnosed as definite DM and eight patients were as possible DM (Fig. 1). All patients had been first classified as PM and DM at the Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, from January 2005 to September 2018. All patients fulfilled the Bohan and Peter criteria for PM and DM and had ever received glucocorticoid and/or immunosuppressive drugs for IIM, which were verified by the Korean Drug Utilisation Review system. All patients underwent neuromuscular examination by neurologists and had the results of anti-Jo-1 and serum muscle enzymes at diagnosis. We reclassified 72 PM patients and 49 DM patients based on the Bohan and Peter criteria by the 2017 EULAR/ACR criteria using an online web calculator (www.imm.Kise/biostatistics/calculators/iim) and investigated the concordance rate of the classification between the two criteria. This study was approved by the Institutional Review Board of Severance Hospital (4-2018-1089), who waived the need for patient written informed consent, as this was a retrospective study.
The mean age of PM patients was 60.5 years old (16 patients were men) and that of DM patients was 54.4 years old (15 patients were men). At diagnosis, proximal muscle weakness was observed in all PM patients but it was not in all DM patients (83.7%). DM-specific typical skin rash was presented only in DM patients but not PM patients at all. By contrast, elevated serum skeletal muscle enzyme levels were detected in all subjects in this study. Electromyography (EMG) and muscle biopsy were performed in PM patients more frequently than DM patients at diagnosis (98.6% vs. 83.7% and 73.3% vs. 51.0%) (Table 1).
When the 2017 EULAR/ACR criteria were applied to 72 PM patients, three patients (4.2%) were newly reclassified as non-IIM due to a total score of 5.3 or smaller. At diagnosis, these three patients were classified as probable PM without muscle biopsy, based on proximal muscle weakness, elevation of serum creatinine phosphokinase (CK) level and abnormal EMG results (Table 2). Meanwhile, one patient, who was classified as possible PM, was newly reclassified as probable PM due to the presence of dysphagia (Table 2).
In addition, when the 2017 EULAR/ACR criteria were applied to 49 DM patients based on Bohan and Peter criteria, eight patients (16.3%) were newly reclassified as ADM due to the absence of proximal muscle weakness. At diagnosis, eight patients were classified as possible DM without muscle biopsy or EMG, based on elevation of serum CK level and DM-specific typical skin rash (Table 2). Nevertheless, these eight patients with DM could clinically be classified as ADM based on the Bohan and Peter criteria, despite no comment on ADM.
The concordance rate of the classification between the Bohan and Peter criteria and the 2017 EULAR/ACR criteria was 95.8% for PM patients and 83.7% for DM patients. Because one patient with possible PM was reclassified as probable PM, one patient was not included in the concordance rate analysis.
In this study, we reclassified Korean patients with PM and DM, who were classified by the Bohan and Peter criteria at diagnosis, the 2017 EULAR/ACR criteria and found that three of 72 PM patients were newly reclassified as non-IIM and eight of 49 DM patients were reclassified as ADM. The concordance rate of the classification between the two criteria was 95.8% for PM patients and 83.7% for DM patients. On the other hand, we compared the concordance rates in IIM patients with and without the results of the muscle biopsy. PM patients with histological results of the muscle biopsy exhibited a concordance rate of 100%, whereas those without the histological results showed a concordance rate of 80.0% between the Bohan and Peter criteria and 2017 EULAR/ ACR criteria. DM patients with the histological results of the muscle biopsy exhibited a concordance of 100%, whereas those without the histological results showed a reduced concordance rate of 52.2% between the two criteria. Therefore, if muscle biopsy could be performed in most cases of PM and DM, the clinical significance of the Bohan and Peter criteria for clearly classifying patients suspected with IIM as PM and DM could be increased. A recent study investigated the relationship between the expert-assigned and the 2017 EULAR/ACR criteria-assigned IIM subtypes. When applying the 2017 EULAR/ ACR criteria to patients with IIM based on the expert opinion, they reported that all 37 patients with PM were reclassified as PM, whereas three of 57 patients with DM were reclassified as PM. The concordance rate of the classification of the recent study was 100% for PM patients and 94.7% for DM patients [12]. However, we could not compare the concordance rate of our study with that of the recent study, because the expert opinion was not according to the Bohan and Peter criteria and furthermore, the geographical and ethnic backgrounds were different.
DM patients without evidence of myositis but with DM-specific typical skin rashes, such as heliotrope rash or GottronŌĆÖs sign or papules based on the Bohan and Peter criteria could be clinically classified as ADM. This assumption may increase the concordance rate in DM patients up to 100%. However, precise attention should be paid to the differential diagnosis ADM from DM, as ADM is a poor prognostic factor for the concurrence of interstitial lung disease in Korean patients with ADM [13]. These overall results suggest that the classification-accuracy of Bohan and Peter criteria might be not inferior to that of the 2017 EULAR/ACR criteria in Korean patients with PM and DM.
A remarkable change between the two criteria was that dysphagia and anti-Jo-1 were included in the 2017 EULAR/ACR criteria. Among several myositis-specific autoantibodies, such as anti-Jo-1, anti-Mi-2, anti-signal recognition particle (SRP), anti-PL-7 and anti-PL-12, only anti-Jo-1 was included [11]. Furthermore, the highest score (3.9 without biopsy and 38 with biopsy) was allocated to the presence of anti-Jo-1. In our study, three PM patients were reclassified as non-IIM by the new criteria. Anti-Jo-1 was not detected and muscle biopsy was not performed in these three patients in whom the classification was changed. Considering the high concordance rate of the classification and the highest score of anti-Jo-1 in the 2017 EULAR/ACR criteria, we suggest that the Bohan and Peter criteria should preferentially be applied to a patient suspected of PM without DM-specific typical skin rash. And then, if the patient is classified as probable PM without both muscle biopsy or anti-Jo-1, performing muscle biopsy should be considered to discern between PM and other IIMs. Meanwhile, although a relatively low score of 0.7 was allocated to dysphagia, which was newly included in the 2017 EULAR/ACR criteria, one patient with possible PM was reclassified as and promoted to probable PM by the new criteria owing to the presence of dysphagia.
Our study has a merit that we first reclassified Korean patients with PM and DM based on the Bohan and Peter criteria at diagnosis by the 2017 EULAR/ACR criteria and demonstrated that the concordance rate between the two criteria was 95.8% for PM patients and 83.7% for DM patients. Our study also has an issue that the number of patients in this study was too small to represent Korean patients due to a retrospective and monocentric study. However, we could minimise the inter-centric variation and provide the unified the neuromuscular examination by neurologists based on the same protocol due to a monocentric study. Future studies with a larger number of IIM patients will afford the more reliable and clearer information on the concordance rate of the classification between the Bohan and Peter criteria and the 2017 EULAR/ACR criteria.
The Bohan and Peter criteria were comparable to the 2017 EULAR/ACR criteria for classifying PM and DM in Korean patients. Given that the Bohan and Peter criteria are convenient in the real clinical settings, we suggest that the old criteria should be preferentially applied and then performing muscle biopsy should be considered in a patient suspected of PM without anti-Jo-1. Moreover, ADM could also clinically be classified by the old criteria.
1. The concordance rate of the classification between the Bohan and Peter criteria and the 2017 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) criteria was 95.8% for polymyositis (PM) patients and 83.7% for dermatomyositis (DM) patients.
2. The Bohan and Peter criteria were comparable to the 2017 EULAR/ACR criteria for classifying PM and DM in Korean patients.
3. The old criteria should be preferentially applied and then performing muscle biopsy should be considered in a patient suspected of PM without antihistidyl tRNA synthetase (anti-Jo-1).
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03029050) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
Table┬Ā1.
Baseline characteristics of patients with PM and DM based on the classification criteria proposed by Bohan and Peter
Table┬Ā2.
Fulfilled items of the Bohan and Peter criteria and the 2017 EULAR/ACR criteria
The Bohan and Peter criteria, the classification criteria for polymyositis and dermatomyositis proposed by Bohan and Peter in 1975.
EULAR/ACR, European League Against Rheumatism/American College of Rheumatology; IIM, idiopathic inflammatory myopathy; CK, creatinine phosphokinase; LDH, lactate dehydrogenase; AST, aspartate transaminase; ALT, alanine transaminase; EMG, electromyography; DM, dermatomyositis; Anti-Jo-1, antihistidyl tRNA synthetase; PM, polymyositis; ADM, amyopathic dermatomyositis.
REFERENCES
1. Tieu J, Lundberg IE, Limaye V. Idiopathic inflammatory myositis. Best Pract Res Clin Rheumatol 2016;30:149ŌĆō168.


3. Callen JP. Cutaneous manifestations of dermatomyositis and their management. Curr Rheumatol Rep 2010;12:192ŌĆō197.



4. Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle Nerve 2015;51:638ŌĆō656.


5. Lundberg IE, Miller FW, Tjarnlund A, Bottai M. Diagnosis and classification of idiopathic inflammatory myopathies. J Intern Med 2016;280:39ŌĆō51.



6. Lundberg IE, Svensson J. Registries in idiopathic inflammatory myopathies. Curr Opin Rheumatol 2013;25:729ŌĆō734.


7. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344ŌĆō347.


8. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403ŌĆō407.


9. Schmidt K, Schmidt J. Inclusion body myositis: advancements in diagnosis, pathomechanisms, and treatment. Curr Opin Rheumatol 2017;29:632ŌĆō638.


10. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med 2016;280:8ŌĆō23.


11. Lundberg IE, Tjarnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955ŌĆō1964.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



