INTRODUCTION
After the first case of coronavirus disease 2019 (COVID-19) was confirmed in Korea in January 2020, the country had the second-highest number of COVID-19 patients in the world until early February 2020 [
1,
2]. As of March 11, 2022, a cumulative total of 5,539,650 cases of COVID-19 had been confirmed in Korea, with a case fatality rate of 0.17% [
3]. Bacterial and fungal superinfections are life-threatening infectious complications in severe respiratory viral infections [
4]. Approximately 5% of patients with COVID-19 are critically ill and require intensive care management; therefore, concerns about superinfections with COVID-19 have persisted. Secondary or co-infection with other respiratory pathogens in severe COVID-19 patients has been reported, and research on COVID-19-associated pulmonary aspergillosis (CAPA) has been documented from the early period of the pandemic [
4–
6].
Previous studies have reported that CAPA occurs in approximately 10% of critically ill COVID-19 patients admitted to the intensive care unit (ICU), with a mortality rate of 54% [
7,
8]. The incidence of CAPA was higher than that of invasive pulmonary aspergillosis (IPA) in critically ill patients without a diagnosis of COVID-19 (1% to 7%), and the mortality rate of CAPA was even higher than that of IPA in patients with hematologic malignancies [
9,
10]. Advanced age, chronic obstructive pulmonary disease (COPD), high Sequential Organ Failure Assessment score, and high Acute Physiology and Chronic Health Evaluation II score have been suggested as risk factors for CAPA [
6,
10–
12].
However, most studies were case series or retrospective studies conducted in Europe with a focus on critical COVID-19 patients. Therefore, regional differences and the varying epidemiology of patients with severe COVID-19 have not yet been fully elucidated. Systemic corticosteroids have been widely used as standard care for severe COVID-19 patients after the clinical study by Randomized Evaluation of COVID-19 Therapy [
13], but their impact on the development of CAPA has not been thoroughly determined [
8,
14]. Furthermore, it remains controversial whether the high mortality rate of CAPA is due to CAPA itself or other factors such as advanced age and comorbidities [
10,
12].
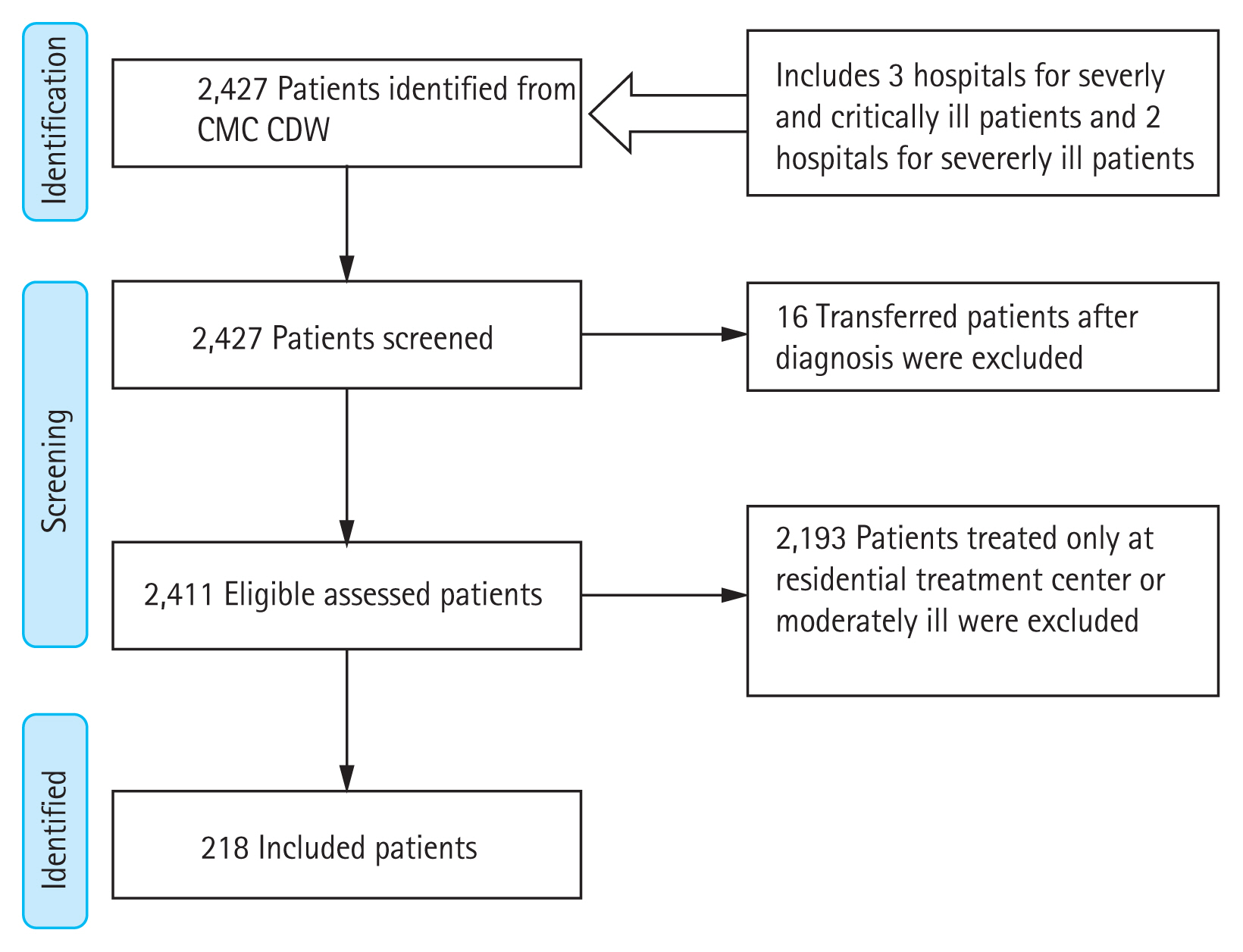
This study aimed to evaluate the epidemiologic trend of CAPA in Korea and clarify the importance of diagnostic efforts. In addition, we conducted this study to identify the predictors of CAPA and risk factors for mortality in patients who were severely to critically ill COVID-19.
DISCUSSION
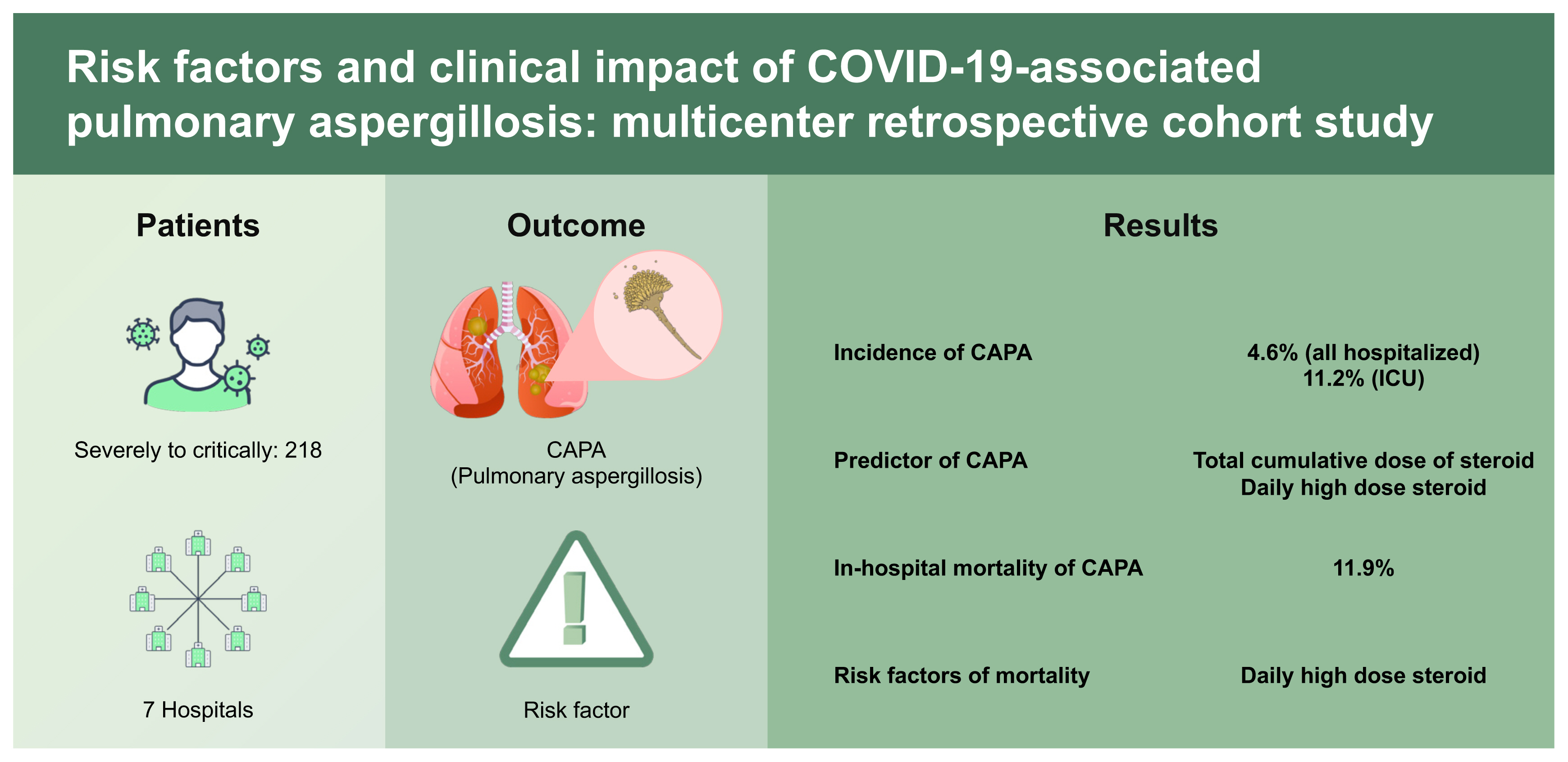
In this retrospective multicenter study, the incidence of CAPA was 4.6% in all hospitalized patients and 11.2% in all ICU patients. We also demonstrated the importance of clinical awareness and surveillance methods for CAPA diagnosis based on the incidence between study hospitals. Systemic use of corticosteroids was the only modifiable predictor for CAPA development (total cumulative dose over 1,000 mg and daily high-dose use over 60 mg) and risk factor (daily high-dose use over 60 mg) for mortality related to COVID-19. However, CAPA itself did not independently affect the outcome. The results of this study highlighted that daily high-dose corticosteroid use for more than 7-day may strongly affect the occurrence of CAPA and the overall mortality. It is mandate to include the superinfections such as CAPA and potential adverse effects of overusing systemic corticosteroid based on accumulated data in the guidelines for COVID-19.
IPA following respiratory viral infection has been reported in patients with influenza, severe acute respiratory syndrome, and adenoviral pneumonia [
20,
21]. In previous studies, the incidence of CAPA in COVID-19 patients admitted to the ICU varied from 3%–39% and 0.7%–7.7% in all hospitalized patients [
7,
10]. This was mainly based on studies conducted in Europe, where experienced medical staff and equipment are readily available for IPA diagnosis. Therefore, it was difficult to estimate the differences according to medical resources and regional differences. The results of this study showed that the incidence of CAPA (4.6% among all hospitalized COVID-19 patients and 11.2% in the ICU) and median time to CAPA diagnosis from admission (9.5 days) were consistent with those of previous studies, and it was difficult to find significant regional differences [
7,
10,
22]. However, the incidence in this study could be underestimated due to a couple of reasons: First, some of the time period included in this study was before the widespread use of immunomodulating agents such as corticosteroids, interleukin 6 (IL-6) monoclonal antibodies, IL-1 receptor antagonists, and Janus kinase inhibitors which are known to affect CAPA occurrence [
7,
23]. Second, there was a lack of awareness of CAPA in the early period of the COVID-19 pandemic, and only one hospital performed a surveillance under high index of suspicion for CAPA in ICU patients.
Although the overall incidence was consistent with previous studies, there was a notable difference in CAPA incidence between hospitals. In the one hospital that performed active surveillance for CAPA, the incidence was similar to that found in previous studies (13.4%, 9/67), but in the other hospitals that did not perform active surveillance, the CAPA incidence was substantially lower (0.6%, 1/151). There are limitations in early diagnosis due to atypical features of imaging tests and difficulties in defining mycological factors such as GM and polymerase chain reaction (PCR) tests [
6,
11,
12]. Therefore, active surveillance has been emphasized in high-risk patients for early diagnosis and treatment of CAPA, but there is no universal surveillance protocol [
16,
22]. In one of the hospitals in this study, active surveillance was performed twice a week with serum GM, BDG, and culture (sputum or tracheal aspirate) for severely and critically ill COVID-19 patients in the ICU receiving oxygen therapy via HFNC or mechanically ventilated. If the test was positive, CAPA was diagnosed after a consultation with an infectious disease specialist based on ECMM/ISHAM consensus criteria. The incidence of CAPA in the hospital that performed active surveillance was similar to that of previous studies, but it showed a distinct difference in other hospitals that did not undergo surveillance, confirming the importance and appropriateness of the surveillance method using serologic tests and culture in severely or critically ill COVID-19 patients [
6,
11,
22,
24].
The cornerstone of the mycological diagnosis of CAPA in the ECMM/ISHAM criteria is culture, GM, and PCR using bronchoalveolar lavage. However, many institutions in this study cannot actively perform bronchoscopy for CAPA diagnosis because of safety issues such as aerosol generation and lack of professional medical staff and equipment [
16,
22]. Bronchoscopy was performed in only 5% of cases in this study, and all mycological evidence for probable CAPA was based on serum GM. Serum GM is readily available in many institutions, and its effectiveness has already been confirmed in influenza-associated pulmonary aspergillosis diagnosis, similar to CAPA [
25,
26]. Since low sensitivity of serum GM was reported due to the lack of angioinvasion of
Aspergillus in non-immunocompromised patients, previous studies suggested the use of serum GM along with serum BDG or PCR results to enhance sensitivity and specificity [
5,
9,
27,
28]. Thus, diagnostic efforts through high index of suspicion with serological tests may be effective supplementary method for diagnosing CAPA in a resource-limited setting [
22,
27,
29].
Monoclonal antibody and remdesivir were widely used in mild to moderate COVID-19 patients, whereas systemic corticosteroids became the standard treatment in severe to critical COVID-19 patients [
14,
30,
31]. Previous studies reported that no significant difference in secondary infections, including CAPA, due to systemic corticosteroid use, but there was still limitation in the analysis between systemic corticosteroid and CAPA development as the type, doses, frequency and duration of corticosteroid administration were not well described [
8]. Furthermore, it was difficult to estimate the effect of long-term and high-dose corticosteroid use in real-world as most of these results were derived from studies with a low-dose use (100 mg as prednisolone in patients with CAPA vs. 107 mg without CAPA in prospective study) or short period of use (dexamethasone 6 to 10 mg up to 10 days in clinical trial) [
13,
32,
33]. In the univariate analysis in this study, age over 60 years, lymphopenia, MV, and comorbidities such as COPD, chronic kidney disease, CLD, and hematologic malignancy had a statistically significant impact on CAPA development, which is consistent with the findings of previous studies [
5,
7,
8]. As predictors of CAPA, the dose and duration of systemic corticosteroids were the only significant and modifiable factors. In multivariate analysis adjusted for covariates, higher total cumulative doses of corticosteroid use and daily high-dose corticosteroid use were significant independent predictors and CAPA only diagnosed when the mean daily dose exceeded 30 mg as methylprednisolone. However, an early high-dose corticosteroid use did not independently affect the occurrence of CAPA. Previous studies have raised concerns about the association between systemic corticosteroid use and the incidence of CAPA [
27]. In the influenza-associated pulmonary aspergillosis study, similar to CAPA, it was confirmed that systemic corticosteroid use is a significant risk factor for the occurrence of IPA in patients with severe influenza [
21]. Therefore, limited use of systemic corticosteroids is recommended in patients with severe influenza, and administration after IPA exclusion through GM is recommended for acute respiratory distress syndrome (ARDS) patients who need corticosteroid use [
21]. In this study, we presented the risk of systemic corticosteroid not only by the total cumulative dose, also the cumulative dose over 7 days, mean daily dose and duration of use. Early use of high-dose steroids due to control hyperinflammatory response with COVID-19 might have minimal effect on the development of CAPA, but high-dose systemic corticosteroid therapy after early inflammatory phase is strongly associated with the development of CAPA. Although, administration of high-dose corticosteroids to control the initial inflammatory response is inevitable, efforts to reduce and stop systemic corticosteroid is important when treating severe COVID-19 patients [
34]. As in previous clinical trial [
13], we recommend not to exceed an mean daily dose of 30 mg/day or less or at least 60 mg/day to prevent CAPA. Furthermore, rigorous surveillance for CAPA is necessary for the patients with prolonged administration of high-dose corticosteroids.
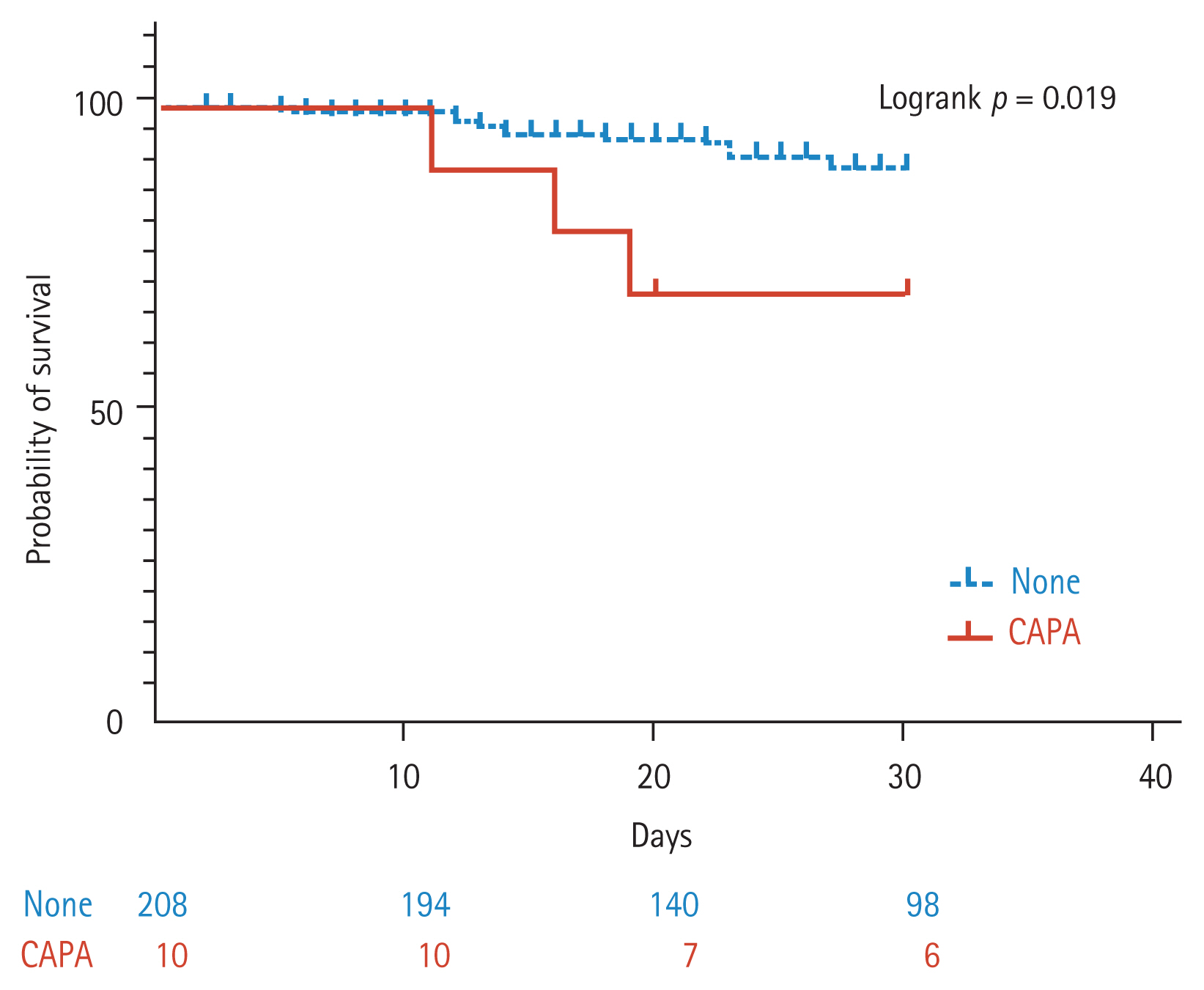
The overall mortality of patients with CAPA was 54.9%, and some studies have reported up to 100%, which was higher than the mortality of IPA in patients with hematologic malignancies [
10]. Previous studies found that CAPA was associated with a 16% to 25% excess in mortality, which is similar to that found in patients with influenza-associated pulmonary aspergillosis [
27,
32,
35]. However, there was a lack of analysis on whether the high mortality rate was the impact of CAPA itself, or whether it was due to age and underlying diseases that affect the development of CAPA [
5,
7,
10]. In this study, the in-hospital overall mortality of patients with CAPA was 50%, and the occurrence of CAPA had a significant impact on 30-day overall survival in univariate analysis. However, the impact of CAPA on mortality only showed an increasing trend in multivariate analysis using the proportional hazard model. In univariate analysis, age over 60 years, COPD, elevated creatinine, CLD, heart failure or coronary artery disease, oxygen therapy and systemic corticosteroid use (total cumulative dose, early high-dose use and daily high-dose use) were associated with high mortality rates, consistent with previous studies [
36–
38]. However, oxygen therapy and daily high-dose of systemic corticosteroids use were identified as independent risk factors for mortality in the multivariate analysis. The survival benefit of using systemic corticosteroids in severely to critically ill COVID-19 patients in previous studies was about 6 mg of dexamethasone (30 mg of methylprednisolone) for 10 days, but the actual use of steroids in clinical practice was higher than this [
8,
13]. This trend seems to be due to results of previous studies indicating that the use of high-dose corticosteroids has improved mortality in ARDS due to COVID-19 [
18]. However, the effect of high-dose corticosteroid use on the survival rate of COVID-19 remains controversial [
19]. In this study, daily high-dose systemic corticosteroid use increased mortality after adjusting for covariates and this was the only modifiable risk factor for mortality but not early high-dose use. In this regard, we suggest that it is necessary to reduce or stop systemic corticosteroid after early inflammatory phase in patients with severe to critical COVID-19 to improve survival.
This study had some limitations. First, although this study was performed based on a multicenter design, confounders could not be fully adjusted due to a limited number of patients with CAPA. Second, we did not evaluate the impact of the delta variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and immunomodulating agents such as IL-6 inhibitors on CAPA development as the delta variant was not a dominant strain and immunomodulators were not widely used during much of the study period. Finally, this study was a retrospective study based on a big-data platform. This makes it difficult to determine whether there is a causal relationship between high-dose corticosteroid use and excess mortality. In addition, it was not possible to analyze the antifungal prescription pattern or the agreement rate of the operational definition by study hospital. Despite these limitations, our study has several strengths. The results reflect the incidence and predictors of CAPA in Korea with a sizable cohort based on clinical data. Second, we highlight the importance of active surveillance and clinical suspicion of CAPA for severely and critically ill COVID-19 patients through comparisons of incidence between study hospitals. Lastly, we identified and specified the impact of systemic corticosteroids on the occurrence and outcome of COVID-19 and CAPA.
In conclusion, CAPA is not uncommon in Korea, and clinical awareness with active surveillance methods for CAPA diagnosis is essential for early diagnosis and treatment especially in ICU patients. The use of systemic corticosteroids is inevitable for the management of severely and critically ill COVID-19 patients in the early inflammatory period, and early high-dose corticosteroid use may be acceptable as it did not significantly influence the occurrence of CAPA and the mortality. However, daily high-dose corticosteroids use after the early inflammatory phase should be avoided to prevent CAPA and reduce mortality.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



