 |
 |
| Korean J Intern Med > Volume 37(5); 2022 > Article |
|
Abstract
Background/Aims
Results
Conclusions
Acknowledgments
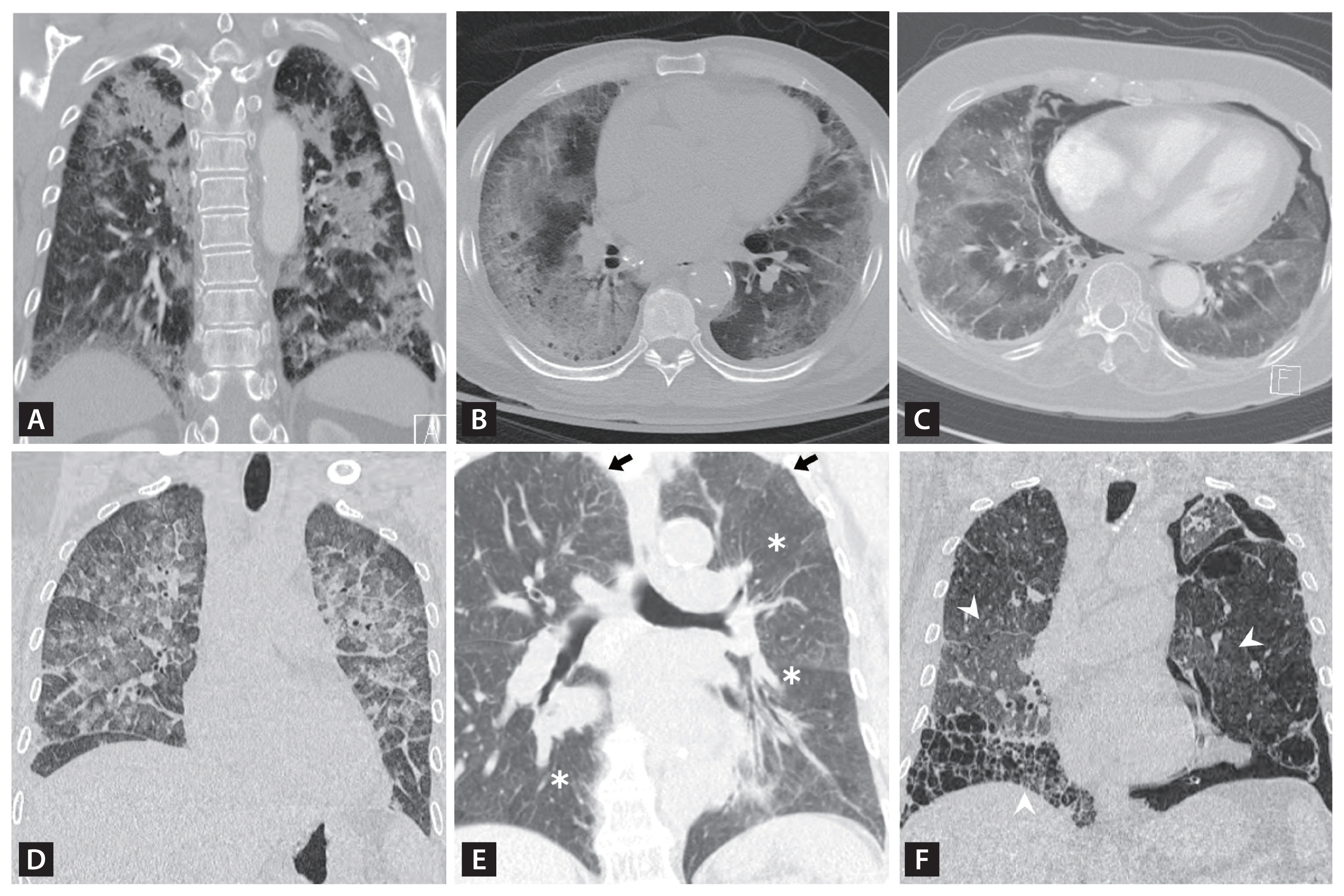
Figure┬Ā1
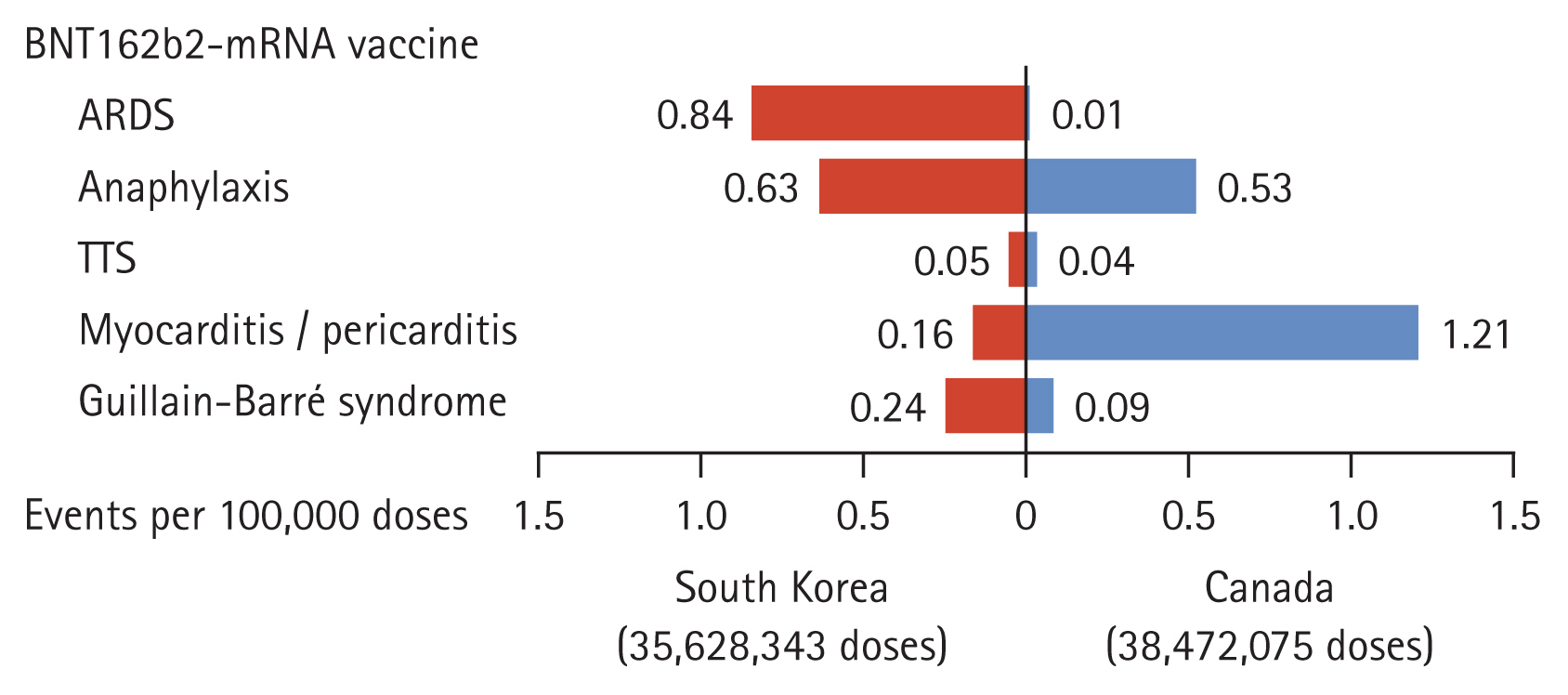
Figure┬Ā2
Table┬Ā1
| New-onset respiratory symptoms after COVID-19 vaccination meeting all of the following criteria: |
| ŌĆāA: Singularity of the agent and temporal eligibility: onset of symptoms and parenchymal opacities on chest computed tomography (CT) must be temporally associated with only COVID-19 vaccination |
| ŌĆāB: Chest CT abnormalitiesa: new occurrence or increased severity of interstitial lung disease (nonspecific interstitial pneumonia, organizing pneumonia, hypersensitivity pneumonitis, simple pulmonary eosinophilia, diffuse alveolar damage [acute respiratory distress syndrome pattern]) |
| ŌĆāC: Exclusion of other diagnoses: COVID-19, infectious pneumonia, embolism, aspiration, inhalations, cardiac failure or fluid overload, sepsis, trauma, and transfusion-related adverse reactions. |
| Bronchoalveolar lavage is recommended when possible. Lung biopsy is not mandatory but could be performed to exclude other diagnoses. |
a The definitions of CT patterns of COVID-19 vaccine-related pneumonitis are described in Supplementary Table 1.
Table┬Ā2
| Characteristic | Total (n = 11) | De novo CV-P (n = 8) | Pre-existing ILD (n = 3) |
|---|---|---|---|
| Demographics | |||
| ŌĆāAge, yr | 80 (51ŌĆō83) | 78 (50ŌĆō84) | 80 |
| ŌĆāMale sex | 5 (45) | 3 (38) | 2 (67) |
| ŌĆāCurrent or past smoker | 4 (36) | 2 (25) | 2 (67) |
| ŌĆāVaccine doses receiveda | m1: 6, m2: 4, a1: 1 | m1: 4, m2: 3, a1: 1 | m1: 2, m2: 1 |
| ŌĆāSymptom onset, dayb | 9 (4ŌĆō13) | 9 (6ŌĆō13) | 7 |
| Comorbid disease | |||
| ŌĆāConnective tissue disease | 0 | 0 | 0 |
| ŌĆāAsthma | 0 | 0 | 0 |
| ŌĆāDiabetes mellitus | 6 (55) | 5 (63) | 1 (33) |
| ŌĆāChronic kidney disease | 1 (9) | 1 (13) | 0 |
| ŌĆāHypertension | 6 (55) | 4 (50) | 2 (67) |
| ŌĆāCardiovascular disease | 1 (9) | 1 (13) | 0 |
| Symptoms | |||
| ŌĆāCough | 10 (91) | 7 (88) | 3 (100) |
| ŌĆāSputum production | 9 (82) | 6 (75) | 3 (100) |
| ŌĆāShortness of breath | 10 (91) | 8 (100) | 2 (67) |
| ŌĆāTemperature > 38┬░C | 5 (45) | 5 (63) | 0 |
| Initial laboratory findings | |||
| ŌĆāWBC > 11,000/mm3 | 2/11 (18) | 1/8 (13) | 1/3 (33) |
| ŌĆāBlood eosinophil > 5% | 3/11 (27) | 2/8 (25) | 1/3 (33) |
| ŌĆāCRP, mg/dL | 7.5 (0.6ŌĆō13.6) | 11.4 (5.2ŌĆō13.7) | 0.2 |
| ŌĆāProcalcitonin > 0.5 ng/mL | 0/9 (0) | 0/8 (0) | 0/1 (0) |
| ŌĆāCreatinine > 1.2 mg/dL | 2/11 (18) | 2/8 (25) | 0/3 (0) |
| ŌĆāALT > 40 IU/L | 3/11 (27) | 3/8 (38) | 0/3 (0) |
| ŌĆāBNP > 100 pg/mL | 1/8 (13) | 1/7 (14) | 0/1 (0) |
| ŌĆāTroponin I > 15.6 pg/mL | 1/8 (13) | 1/7 (14) | 0/1 (0) |
| ŌĆāFeNO > 25 ppb | 0/3 (0) | 0/1 (0) | 0/2 (0) |
| Bronchoalveolar lavage | 5 (45) | 5 (63) | 0 |
| Autoantibodies | |||
| ŌĆāAntinuclear antibodies | 4/11 (36) | 3/8 (38) | 1/3 (33) |
| ŌĆāAnti-Ro/SSA | 2/11 (18) | 2/8 (25) | 0/3 (0) |
| ŌĆāAnti-La/SSB | 0/11 (0) | 0/8 (0) | 0/3 (0) |
| ŌĆāRheumatoid factor | 1/11 (9) | 1/8 (13) | 0/3 (0) |
| ŌĆāAnti-CCP | 0/11 (0) | 0/8 (0) | 0/3 (0) |
| Case management | |||
| ŌĆāOxygen supply, FiO2% | 48 (21ŌĆō80) | 65 (44ŌĆō80) | 21 |
| ŌĆāHospitalization | 8 (73) | 7 (88) | 1 (33) |
| ŌĆāSystemic steroid treatment | 11 (100) | 8 (100) | 3 (100) |
| ŌĆāSteroid dose, mg/dayc | 32 (8ŌĆō70) | 57 (28ŌĆō80) | 8 |
| ŌĆāSteroid tapering or withhold | 10/11 (91) | 7/8 (88) | 3/3 (100) |
| ŌĆāFollow-up, mo | 5.2 (3.9ŌĆō8.0) | 4.5 (3.2ŌĆō7.8) | 7.4 |
| ŌĆāDeath | 1 (9) | 1 (13) | 0 |
CV-P, COVID-19 vaccine-related pneumonitis; ILD, interstitial lung disease; WBC, white blood cell; CRP, C-reactive protein; ALT, alanine aminotransferase; BNP, brain natriuretic peptide; FeNO, fractional exhaled nitric oxide (before systemic steroid); Anti-CCP, anti-cyclic citrullinated peptide antibody.
Table┬Ā3
| Characteristic | Pt #1 | Pt #2 | Pt #3 | Pt #4 | Pt #5 | Pt #6 | Pt #7 | Pt #8 | Pt #9 | Pt #10 | Pt #11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | 86 | 83 | 85 | 48 | 82 | 73 | 51 | 44 | 80 | 82 | 80 |
| Sex | Male | Female | Female | Male | Female | Male | Female | Female | Female | Male | Male |
| Vaccine (doses) receiveda | m1 | m2 | m1 | a-m1 | m2 | a1 | m1 | m2 | m2 | m1 | m1 |
| Time from most recent vaccination to symptom onset, day | 1 | 13 | 8 | 9 | 13 | 9 | 4 | 25 | 7 | 12 | 4 |
| Underlying interstitial lung disease | N | N | N | N | N | N | N | N | HP | AEF | IPF |
| Radiologic patterns on chest CT | OP | OP | OP | OP | DAD | DAD | DAD | DAD | A/E | A/E | A/E |
| SARS-CoV-2 PCR test, negativeb | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Current or past smoker | N | N | N | Y | N | Y | N | N | N | Y | Y |
| Comorbid disease | |||||||||||
| ŌĆāConnective tissue disease | N | N | N | N | N | N | N | N | N | N | N |
| ŌĆāAsthma | N | N | N | N | N | N | N | N | N | N | N |
| ŌĆāDiabetes mellitus | Y | Y | Y | N | N | Y | N | Y | N | Y | N |
| ŌĆāChronic kidney disease | Y | N | N | N | N | N | N | N | N | N | N |
| ŌĆāHypertension | Y | N | Y | N | Y | Y | N | N | Y | N | Y |
| ŌĆāCardiovascular disease | N | N | Y | N | N | N | N | N | N | N | N |
| Symptoms | |||||||||||
| ŌĆāCough | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| ŌĆāSputum production | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| ŌĆāShortness of breath | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| ŌĆāTemperature > 38┬░C | Y | N | N | Y | Y | Y | N | Y | N | N | N |
| Initial laboratory findings | |||||||||||
| ŌĆāWhite cell count, /mm3 | 11.6 | 10.2 | 8.4 | 7.0 | 9.1 | 9.7 | 6.0 | 8.7 | 4.7 | 6.7 | 16.9 |
| ŌĆāBlood eosinophil, % | 4.1 | 1.9 | 3.3 | 7.1 | 0.4 | 2.0 | 8.5 | 1.6 | 5.2 | 1.5 | 2.3 |
| ŌĆāC-reactive protein, mg/dL | 11.4 | 13.6 | 2.9 | 14.9 | 1.0 | 11.3 | 7.5 | 13.8 | 0.2 | 0.0 | 0.6 |
| ŌĆāProcalcitonin, ng/mL | 0.32 | 0.01 | 0.06 | 0.33 | 0.15 | 0.22 | 0.08 | 0.08 | NP | NP | 0.03 |
| ŌĆāCreatinine, mg/dL | 1.85 | 0.65 | 0.75 | 0.86 | 0.61 | 1.78 | 0.72 | 0.60 | 0.71 | 0.72 | 0.78 |
| ŌĆāAlanine aminotransferase, IU/L | 11 | 12 | 12 | 57 | 70 | 37 | 64 | 18 | 22 | 12 | 21 |
| ŌĆāBrain natriuretic peptide, pg/mL | 88 | 32 | NP | 139 | 29 | 16 | 24 | 10 | NP | NP | 1 |
| ŌĆāTroponin I, pg/mL | 15.2 | < 10 | NP | 11.4 | 28.4c | < 10 | < 10 | < 10 | NP | NP | 10.5 |
| ŌĆāFeNO, ppb, before systemic steroids | NP | NP | NP | NP | NP | 24 | NP | NP | 9 | NP | 18 |
| Bronchoalveolar lavage | NP | Y | NP | Y | NP | Y | Y | Y | NP | NP | NP |
| Autoantibodies, positive | |||||||||||
| ŌĆāAntinuclear antibodiesd | N | N | Y | N | Y | Y | N | N | Y | N | N |
| ŌĆāAnti-Ro/SSA antibody | N | N | N | N | Y | Y | N | N | N | N | N |
| ŌĆāAnti-La/SSB antibody | N | N | N | N | N | N | N | N | N | N | N |
| ŌĆāRheumatoid factor | N | N | N | N | N | Y | N | N | N | N | N |
| ŌĆāAnti-citrullinated peptide antibody | N | N | N | N | N | N | N | N | N | N | N |
| Case management | |||||||||||
| ŌĆāOxygen supply, FiO2, % | 50 | 40 | NP | 48 | 100 | 80 | 80 | 80 | NP | NP | 45 |
| ŌĆāHospitalization | Y | Y | N | Y | Y | Y | Y | Y | N | N | Y |
| ŌĆāSystemic steroid treatment | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| ŌĆāSteroid dose, mg/day (MPD equivalent) | 63 | 24 | 12 | 32 | 125 | 70 | 90 | 50 | 8 | 8 | 8 |
| ŌĆāSteroid tapering or withhold | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| ŌĆāTime from onset to last follow-up, mo | 11.7 | 8.0 | 7.6 | 3.9 | 1.1 | 4.6 | 2.5 | 4.3 | 7.4 | 5.2 | 9.6 |
| ŌĆāDeath | N | N | N | N | Y | N | N | N | N | N | N |
Pt, patient; HP, hypersensitivity pneumonitis; AEF, airspace enlargement with fibrosis; IPF, idiopathic pulmonary fibrosis; CT, computed tomography; OP, organizing pneumonia; DAD, diffuse alveolar damage; A/E, aggravation or exacerbation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PCR, polymerase chain reaction; NP, not performed; FeNO, fractional exhaled nitric oxide; MPD, methylprednisolone.
a m: BNT162b2-mRNA vaccine, a: ChAdOx1 nCoV-19. a-m: The first dose was ChAdOx1 nCoV-19, and the second dose was the BNT162b2-mRNA vaccine. This is due to a temporary change in the age criteria for ChAdOx1 nCoV-19 in Korea, not for patientŌĆÖs medical reasons.
b Real-time PCR coronavirus disease 2019 (COVID-19) test was performed (Real-Q 2019-nCoV Detection kit, BioSewoom, Seoul, Korea).
Table┬Ā4
| Characteristic | Case #1 | Case #2 | Case #3 | Case #4 | Case #5 | Case #6 | Case #7 | Case #8 | Case #9 | Case #10 | Case #11 | Case #12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case reporting country | Japan | Japan | Japan | Japan | Japan | Japan | Spain | Australia | Japan | Japan | United States | Morocco |
| Age, yr | 66 | 85 | 62 | 66 | 65 | 60 | 37 | 55 | 83 | 65 | 40 | 66 |
| Sex | Male | Male | Male | Male | Male | Male | Male | Female | Male | Male | Male | Male |
| Vaccine (doses) receiveda | m1 | m1 | m2 | m2 | m1 | m2 | m2 | m2 | m1 | m2 | m2 | a1 |
| Time from most recent vaccination to symptom onset, day | 1 | 4 | 2 | 5 | 3 | 2 | 1 | 4 | 1 | 6 | 1 | 1 |
| Underlying interstitial lung disease | DIP | ILA | N | N | N | N | Unknown | Unknown | IIP | IIP | Unknown | Unknown |
| Current or past smoker | Y | Y | N | Unknown | Y | Y | Unknown | Unknown | Y | Y | Y | Unknown |
| Symptoms | ||||||||||||
| ŌĆāCough | N | N | N | Y | Y | N | Y | Y | N | N | Y | N |
| ŌĆāSputum production | N | N | N | N | N | N | N | N | N | N | Y | N |
| ŌĆāShortness of breath | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| ŌĆāFever | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Bronchoalveolar lavage | Y | Y | NA | NA | Y | Y | Y | NA | NA | NA | NA | NA |
| CT features and lesion distribution | ||||||||||||
| ŌĆāRadiologic patterns | NSIP | NSIP | OP | DAD | DAD | DAD | DAD | OP | NSIP | DAD | OP | OP |
| ŌĆāBilateral distribution | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| ŌĆāDiffuse distribution | Y | N | N | Y | Y | Y | Y | N | N | Y | N | N |
| ŌĆāMultifocal distribution | N | Y | Y | N | N | N | N | Y | Y | N | Y | Y |
| ŌĆāSubpleural distribution | N | Y | Y | N | N | N | N | N | N | N | N | N |
| ŌĆāConsolidation | Y | N | Y | N | N | Y | N | Y | Y | Y | N | Y |
| ŌĆāGround-glass opacity | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| ŌĆāInterstitial thickening | Y | Y | N | Y | Y | Y | Y | N | Y | Y | N | N |
| Case management | ||||||||||||
| ŌĆāSystemic steroid treatment | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y |
| ŌĆāSteroid dose, mg/day (MPD equivalent) | None | 1,000 | 16 | 1,000 | 1,000 | 1,000 | None | 20 | Pulseb | Pulseb | 40 | 240 |
| ŌĆāDeath | N | N | N | N | N | N | N | N | N | N | N | N |
Case #1-#3, Shimizu et al. [25]; Case #4, Kono et al. [26]; Case #5, Matsuzaki et al. [27]; Case #6 Yoshifuji et al. [28]; Case #7, Piqueras et al. [29]; Case #8, Stoyanov et al. [30]; Case #9 and #10, Amiya et al. [31]; Case #11, Wang et al. [32]; Case #12 Miqdadi et al. [33].
COVID-19, coronavirus disease 2019; DIP, desquamative interstitial pneumonia; ILA, interstitial lung abnormality; IIP, idiopathic interstitial pneumonia; NA, not available; CT, computed tomography; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; DAD, diffuse alveolar damage; MPD, methylprednisolone.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



