 |
 |
| Korean J Intern Med > Volume 40(5); 2025 > Article |
|
Abstract
Background/Aims
Although COVID-19 vaccines reduce COVID-19 severity, various safety concerns have emerged. This study, involving a population-based cohort, used health insurance data to investigate potential vaccine-related major outcomes, including cardiac, pulmonary, and thromboembolic diseases.
Methods
This retrospective cohort study involved data from 2,017,884 vaccinated (at least two doses) individuals and 334,583 unvaccinated individuals. The incidences of myocarditis, myocardial infarction, atrial fibrillation, interstitial lung disease, pulmonary thromboembolism, deep vein thrombosis, and cerebrovascular disease were compared between the vaccinated and the unvaccinated groups at 1 week to 3 months after vaccination.
Results
The study population had a mean age of 54 years (male: 44.6%). Among the vaccinated, 57.7% received the mRNA vaccine only, whereas 35.5% received the adenoviral vector vaccine alone. Multivariate logistic analysis revealed that vaccination was significantly associated with the early development of myocarditis. The mRNA vaccine, a younger age, and hyperlipidemia were independent indicators of poor myocarditis prognosis after vaccination. However, the incidence of myocardial infarction at 1–2 weeks post-vaccination, as well as pulmonary thromboembolism and cerebrovascular disease (both at 3 months post-vaccination), were significantly lower in the vaccinated group when compared with the unvaccinated one. However, there was no significant association between vaccination and interstitial lung disease, atrial fibrillation, or deep vein thrombosis.
Conclusions
The younger male population (age: < 45 years) should be cautious about receiving the COVID-19 mRNA vaccine and should be closely monitored for myocarditis after vaccination. Vaccination was associated with short-term protection against venous and arterial thrombotic events, as well as hemorrhagic events.
Following the declaration of the coronavirus disease-19 (COVID-19) pandemic in 2019, numerous vaccines, including adenoviral vector vaccines (e.g., AZD1222 and Ad26. COV2.S) and mRNA vaccines (BNT162b2 and mRNA-1273) were rapidly developed. Although these vaccines are all based on the viral spike (S) protein antigen, their modes of antigen presentation to the immune system vary [1]. Early COVID-19 studies indicated that the efficacies of these vaccines for COVID-19 prevention ranged from 66% to 95% [2–6], leading to their authorization for global administration for emergency use or their approval by the United States Food and Drug Administration [7].
Although phase 3 trials showed that these vaccines were tolerable when compared with placebo [3,4,8], safety concerns about their risk arose because of reports about the occurrence of various conditions, including myocarditis, vaccine-induced immune thrombotic thrombocytopenia, and Guillain–Barre syndrome following vaccination [9–11]. Indeed, potential age- and gender-associated safety concerns about vaccination were highlighted by real-world cohort studies [12–14]. However, these studies did not compare the incidence rates of the complications with those observed in unvaccinated individuals during the COVID-19 pandemic. Therefore, a definite association between the vaccine and these adverse events is not established.
Recently, our team reported various vaccine-related adverse events in a population-based cohort from Seoul derived from national health insurance data [15,16]. Here, we investigated the incidence of major potential vaccine-associated events during the short-term period (≤ 3 months), such as cardiac diseases (e.g., myocarditis and arrhythmia), respiratory diseases (e.g., interstitial lung disease [ILD]), and arterial or venous thrombotic events, as well as their prognostic factors in vaccinated vs. unvaccinated individuals.
This retrospective cohort study is based on the Korea National Health Insurance Database. As of January 1, 2021, about 50% of Seoul residents were randomly selected for inclusion in the study. The study group was then analyzed to determine the incidence of vaccination-related major events of interest at 1 week to 3 months after vaccination based on the International Classification of Diseases 10th Revision (ICD-10) codes (Supplementary Table 1). Individuals who received two or more COVID-19 vaccinations were defined as vaccinated and the date of their second vaccination (before September 30, 2021) was recorded as their index date. For non-vaccinated individuals, September 30, 2021, was recorded as the index date. Individuals who received only one vaccination dose were excluded from the analysis. Next, the diagnostic records of the year before the index date were reviewed, and individuals with vaccination-related major events as a primary or secondary diagnosis were excluded from further analysis. Cases in which such events were recorded as the primary diagnosis after the index date were defined as event occurrences.
Ethical approval for the study was granted by the institutional review board of Ewha Womans University Mokdong Hospital (IRB No. 2022-07-003). The study adhered to the Declaration of Helsinki guidelines. The requirement for written informed consent was waived because this is a retro spective study.
The records of the randomly selected patients were reviewed to retrieve data on demographics (e.g., age and gender), vaccination status and type, primary and secondary diagnoses (from 2020–2021), hospital visit dates, underlying diseases, and COVID-19 history.
The following covariates were used for multivariate analysis: age, gender, insurance premium grade, Charlson’s comorbidity index, and the presence of diabetes, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and prior COVID-19 infection (Supplementary Table 2). Insurance premiums were categorized into the low, middle, and high grades based on the health insurance premium. The selection of diagnostic codes for each disease based on Charlson’s comorbidity index was based on the previous study by Sundararajan et al. [17]. The presence of diseases included in the Charlson’s comorbidity index, as well as diabetes, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease, were defined based on whether they were registered as primary or secondary diagnoses more than twice in the year before the index date. COVID-19 infection was defined as the presence of the ICD-10 code, U071, as the primary or secondary diagnosis before the index date.
The student t-test was used to compare continuous variables. Categorical variables were compared using the Chi-square test or Fisher’s exact test. The incidence rate was calculated per 10,000 persons. A logistic regression model was used to calculate the odds ratio with the corresponding 95% confidence interval (95% CI) and p value. The Cox regression model was used to calculate the hazard ratio (HR) with the corresponding 95% CI and p value. If an individual died without experiencing the event of interest, the observation was treated as censored at the time of death. No missing data were present in the analytic dataset. p < 0.05 indicated statistically significant differences. All statistical analyses and data curation were done on the SAS Enterprise Guide (SAS Institute, Cary, NC, USA).
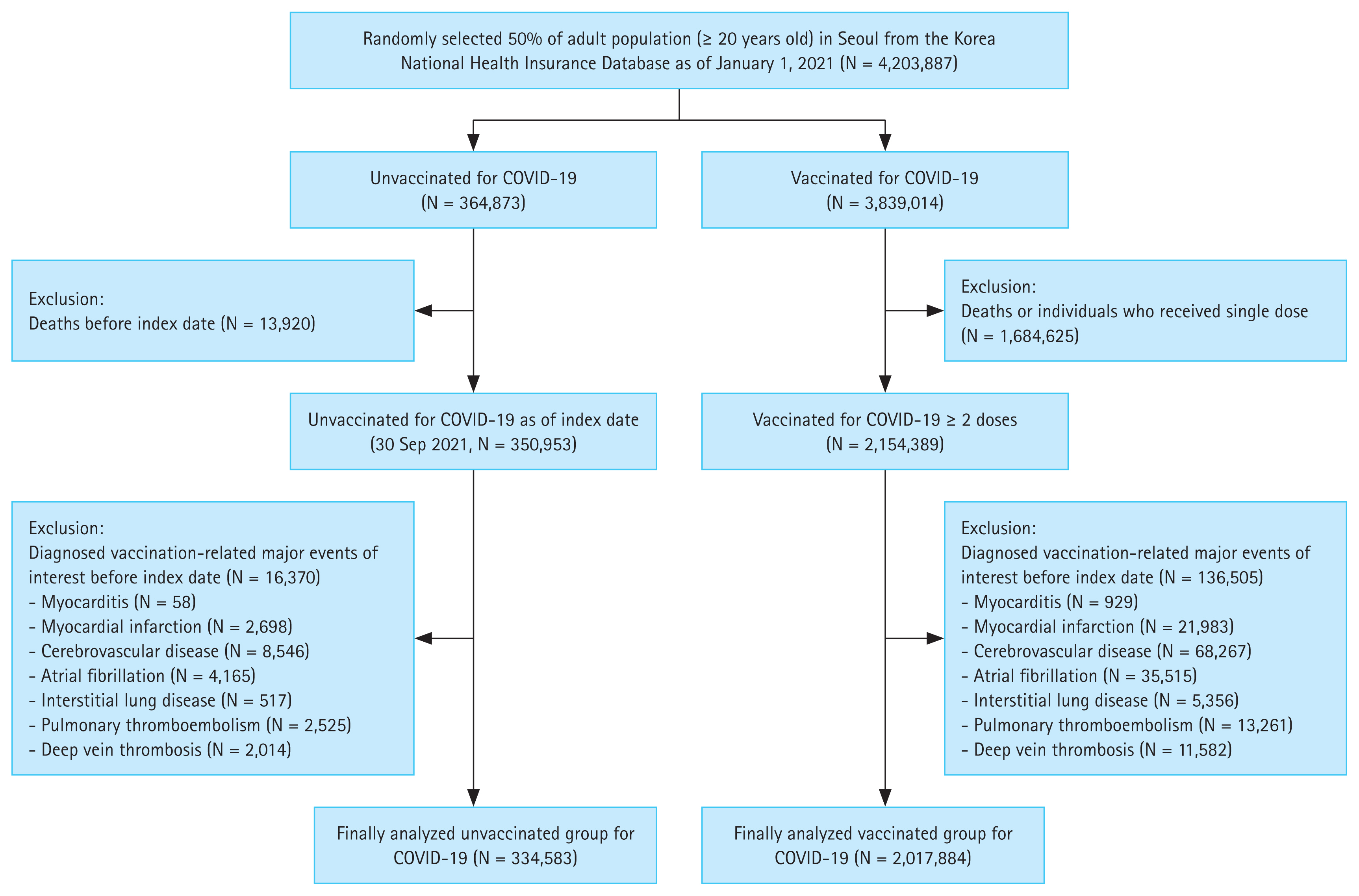
Out of the randomly selected study group (50% of the Seoul population), there were 4,203,887 adults aged ≥ 20 (Fig. 1). Of these, 2,154,389 had received the second dose of the COVID-19 vaccine as of September 30, 2021, whereas 350,953 remained unvaccinated. After excluding individuals who had been diagnosed with the major events of interest before the index date, the final study group involved data from 2,017,884 vaccinated individuals and 334,583 unvaccinated individuals.
The entire study population had a mean age of 54 years and 44.6% of the population was male. Before the index date, 0.74% of the study group had been diagnosed with COVID-19 (Table 1). When compared with the unvaccinated group, the vaccinated group was older, with a higher proportion of females, higher insurance levels, and a higher rate of underlying health conditions. Of the vaccinated group, 57.7%, 35.5%, and 6.8% had received the mRNA vaccine only, the adenoviral vector vaccine only, and heterologous vaccines, respectively.
Figure 2 presents the cumulative incidence of major adverse events of interest stratified by vaccination status. The incidence of myocarditis was consistently higher in the vaccinated group across all time points. Additionally, the vaccinated group exhibited higher 3-month incidence rates of myocardial infarction (MI), atrial fibrillation (AF), ILD, and cerebrovascular disease compared with the unvaccinated group. Detailed incidence rates are provided in Supplementary Table 3, and rates stratified by vaccine type are shown in Supplementary Figure 1.
To comprehensively evaluate these associations, we conducted both cumulative incidence (logistic regression) and time-to-event (Cox regression) analyses, with results presented in Figures 3 and 4, respectively. The multivariate logistic analysis demonstrated that vaccination was significantly associated with an increased risk of myocarditis from 1 week to 3 months post-vaccination (Fig. 3).
Although the incidence of MI and cerebrovascular disease appeared higher in the vaccinated group at 3 months (Fig. 2), multivariate logistic analysis revealed that vaccination was associated with a lower risk of MI (1–2 weeks post-vaccination), pulmonary thromboembolism (PTE), and cerebrovascular disease at 3 months (Fig. 3).
While AF and ILD also showed higher unadjusted incidence in the vaccinated group (Fig. 2), multivariate logistic analysis did not confirm significant associations (Fig. 3).
Similarly, multivariate Cox analysis revealed that vaccination was significantly associated with the development of myocarditis (HR: 3.919) and that it was associated with favorable effects on lower rates of PTE (HR: 0.551) and cerebrovascular disease (HR: 0.783) (Fig. 4).
Because myocarditis was the only adverse event observed in the vaccinated group, we analyzed its prognostic factors. The risk of developing myocarditis in the group that received the mRNA vaccine was 5.62-fold higher compared with the group that received only the adenoviral vaccine (p < 0.001). Furthermore, younger age and hyperlipidemia were identified as independent risk factors for the development of myocarditis following vaccination in the multivariate Cox analysis (Supplementary Table 4). Gender was not found to be a significant risk factor in this analysis. However, subgroup analysis based on age and gender revealed that the incidence of myocarditis was only higher in vaccinated younger males when compared with unvaccinated counterparts (Supplementary Table 5).
To the best of our knowledge, this study is the first to investigate the potential impact of vaccination on major events by comparing vaccinated and unvaccinated individuals during the same pandemic period, using a large, population-based cohort. Our analysis found that the rate of myocarditis was significantly increased in patients who received the mRNA vaccines. Additionally, younger age and comorbid hyperlipidemia were identified as independent risk factors for myocarditis after vaccination. However, the risk of MI (at 1–2 weeks after vaccination) and PTE and cerebrovascular disease (both at 3 months after vaccination) were significantly lower in vaccinated individuals, and there was no significant association between vaccination and ILD, AF, or deep vein thrombosis.
Although the unvaccinated group included a higher proportion of young males, multivariate analysis revealed an increased risk of myocarditis following vaccination, consistent with previous reports. Since the start of COVID-19 vaccinations, there have been concerns about the risk of myocarditis following the administration of mRNA vaccines [9,18,19]. Using a population-based cohort study, Anders et al. showed that when compared with the unvaccinated, the mRNA-1273 vaccine was associated with an increased risk of myocarditis (adjusted HR: 3.92, 95% CI: 2.30–6.68) [20]. A recent meta-analysis of 11 studies also showed that the BNT162b2 and mRNA-1273 vaccines were associated with an increased incidence of pericarditis or myocarditis (relative risk [RR] = 2.19, 95% CI: 1.46–3.29 and RR = 4.15, 95% CI: 1.87–9.22, respectively) [21]. Following mRNA vaccination, myocarditis can occur through various mechanisms, such as the activation of proinflammatory cascades after detection of the mRNA vaccine as an antigen, vaccine-induced spike protein IgG antibodies that cross-react with myocardial proteins, mRNA immune reactivity, and cellular immunity to +1 ribosomal frameshifting [22–24].
The multivariate Cox analysis of risk factors for myocarditis revealed that the condition was more prevalent in younger individuals, with a significantly higher incidence observed in vaccinated males aged 30–45 years than their unvaccinated counterparts. Previous studies have also reported that COVID-19 vaccination-associated myocarditis was more prevalent in young, male adults (aged between teens to the 30s) who had received more than two vaccine doses [19,23,25]. A study investigating the incidence of myocarditis in vaccinated Koreans found that those aged 12–17 years were the most frequently affected and sudden deaths because of COVID-19 vaccination-related myocarditis mainly occurred in those aged < 45 years [26]. It is hypothesized that this is because of sex hormone differences, whereby testosterone suppresses anti-inflammatory cells, whereas estrogen inhibits proinflammatory T cells [23]. In this study, we compared the vaccinated population with the unvaccinated group within the same period and found that the incidence of myocarditis was significantly higher in males aged up to 45 years. This suggests that post-vaccination myocarditis should be a concern not only for those in their 10s and 20s but also for the young to mid-adult population aged under 45 years.
It should be noted that our analysis found that vaccinated patients had lower risks of PTE, MI, and cerebrovascular disease when compared with the unvaccinated group. To date, numerous studies have assessed the association between venous thromboembolism (VTE) and viral vector vaccines. The epidemiological study by Andrews et al. [27] found a significant increase in the rate of VTE in individuals aged < 65 years, who had received the AZD1222 vaccine within a month of the first dose when compared with unvaccinated individuals. Bikdeli et al. [28] also showed that the incidence of venous thrombosis within 30 days of vaccination was higher in those who received a viral vector vaccine when compared with historical incidence. Arterial thrombosis [10,29] and cerebrovascular disease [30] have also been reported following vaccination. These observations are hypothesized to be caused by the spike antigen- and the adenoviral vector-associated thrombotic thrombocytopenia [31]. The mechanism underlying the hypothesized effect may involve the generation of PF4-polyanion autoantibodies, the infiltration of megakaryocytes by adenoviral vectors, the expression of the spike protein on platelet surfaces, and the direct activation of platelets and endothelial cells by the adenoviral vector [32].
Some studies have reported opposing findings, with some indicating that mRNA vaccines are not associated with venous and arterial thrombotic events, ischemic stroke, or MI [33,34]. Some studies have also suggested no association between adenoviral vector vaccines and thrombotic events. A phase 3 clinical trial involving Ad26.COV2.S observed only one VTE event in the study group, a rate that was equivalent to that observed in the control group [5]. Houghton et al. [35] compared the incidence of VTE before and after vaccination in a cohort of 792,010 patients, including 48,453 individuals who received the adenoviral vector vaccine, and found no overall increase in the risk of VTE, especially in the adenoviral vector vaccine subgroup (adjusted HR: 0.97, 95% CI: 0.63–1.50). Interestingly, Simpson et al. [36] also reported that those vaccinated with mRNA vaccines had significantly fewer VTE events compared with unvaccinated, suggesting the vaccines may prevent thrombosis (adjusted RR = 0.50, 95% CI: 0.40–0.62). Moreover, some studies indicate that the vaccines also prevented arterial thrombosis [36,37], including stroke, MI, and hemorrhagic cerebrovascular disease [38].
Here, we find that although onset times differed, COVID-19 vaccination exhibited protection against PTE, MI, and cerebrovascular disease for at least 3 months. Although there is no hypothesis explaining this mechanism, the following possibility can be considered. It has been suggested that COVID-19 infection increases the risk of thrombosis [39,40]. Therefore, COVID-19 vaccination may reduce the risk of COVID-19-associated thrombosis. Xie et al. [41] reported that regardless of the type of vaccine, being unvaccinated or partially vaccinated significantly increased the risk of VTE in ambulatory COVID-19 patients when compared with being fully vaccinated (HR: 5.50, 95% CI: 3.00–10.08). Furthermore, Zisis et al. [38] found that the COVID-19 vaccine significantly reduced the risk of VTE and cerebrovascular disease 28 and 90 days after infection. Although we adjusted for COVID-19 patients in our multivariate analysis, this adjustment may not have been effective because of asymptomatic infections, which may not have been tested. Previous findings indicate that the asymptomatic infection rate during a period similar to when our study was conducted ranged from 20–80% [42]. Thus, in the vaccinated group, the incidence of thrombosis might have appeared to decrease because of asymptomatic COVID-19 infections. Hence, the interpretation of these results should be considered with caution.
We also investigated the relationships between vaccination and ILD or AF, which are currently unclear. Although the potential risk of ILD development after vaccination has been suggested by case series [43,44], no large-scale studies have established this association. There is controversy about whether AF occurs after vaccination, and the results have been heterogeneous [45,46]. In this study, although the incidences of ILD and AF were higher in the vaccinated group than in the unvaccinated group at 3 months after vaccination, multivariate analysis did not reveal significant differences. Further research on the long-term effects of vaccination on these conditions will be necessary.
However, this study has some limitations. First, because it relied on national insurance data, the analyses of the major events of interest and COVID-19 infection were based on diagnostic codes only. Because diagnosis assignments may differ between physicians, incidence rates might be underestimated. Additionally, ICD codes could have limited accuracy in identifying medical conditions in the absence of disease-specific imaging or procedure codes [47]. Selection bias or reporting bias may also exist, particularly in the unvaccinated group, owing to disparities in healthcare-seeking behavior. However, our strategy of using diagnostic codes might be the most efficient method for real-world, large-scale cohort studies. Future studies should incorporate more rigorous, disease-specific definitions and account for alternative causes of medical conditions (e.g., viral infections, medication-induced effects) by utilizing complementary data sources. A history of COVID-19 infection may also need to be validated using serological data or more comprehensive infection surveillance beyond ICD codes.
Secondly, the major events that were analyzed may exhibit heterogeneity. However, based on previous studies, by focusing on diseases that the vaccine could potentially influence, we obtained efficient and prompt results. Third, this study involved Asian adults. Because COVID-19 complications and the incidences of adverse events after vaccination may vary by race and age [19,48], further research involving different populations and those aged < 18 years old is necessary. In addition, only individuals who were fully vaccinated with two or more doses were included in the vaccination group, consistent with previous studies [2,4,8,20]. The vaccine products were also heterogeneous. Since major events may occur after a single dose, future studies should include partially vaccinated individuals and assess outcomes based on the specific vaccine products used. The index date was not exactly matched between the vaccinated and unvaccinated groups. Due to the scale and structure of the population-based dataset, individual-level date matching was not feasible within the scope of this study. However, because COVID-19 vaccination was implemented over a relatively short period during the pandemic, the bias introduced using a common index date for the unvaccinated group is likely minimal. Furthermore, baseline health status differed between the vaccinated and unvaccinated groups. During the early phase of Korea’s national vaccination program, individuals with underlying conditions were prioritized for vaccination. To mitigate this potential bias, we performed multivariable adjustments for baseline characteristics and comorbidities. Finally, this study only analyzed the short-term effects of vaccination on major events. This limitation arose because, at the planning stage, only a limited amount of time had elapsed since the commencement of the vaccination campaign. Therefore, the time frames were selected based on biological plausibility and evidence from prior literature [2,19,27,33]. We are currently analyzing the long-term effects of vaccination, and our findings will be updated in the future.
In conclusion, this population-based cohort study investigated the incidence and risk factors of potential vaccine-associated major events in vaccinated vs. unvaccinated individuals. We confirmed the short-term effects of COVID-19 vaccines on major cardiac, pulmonary, and thromboembolic disease events. Our findings show that COVID-19 vaccination was significantly associated with the early development of myocarditis. Notably, mRNA vaccines, a younger age, and hyperlipidemia were risk factors for myocarditis. The vaccine also demonstrated short-term protection against venous and arterial thrombotic events, as well as hemorrhagic events. We recommend carefully weighing the risks and benefits of vaccination based on individual patient conditions. After vaccination, individuals at a higher risk for myocarditis, such as young male adults (< 45 years old) should be closely monitored or considered for personalized vaccination strategies.
1. This study evaluated the incidence of COVID-19 vaccine-related major events.
2. Vaccination was associated with the development of myocarditis, especially with mRNA vaccines.
3. Vaccination significantly lowered the risk of MI, PTE, and cerebrovascular disease.
4. There was no significant association between vaccination and ILD, atrial fibrillation, or DVT.
Notes
CRedit authorship contributions
Myeong Geun Choi: conceptualization, methodology, investigation, data curation, writing - original draft, writing - review & editing; Min-Ho Kim: methodology, investigation, data curation, formal analysis, writing - review & editing; Eun Mi Chun: conceptualization, methodology, investigation, data curation, writing - review & editing
Figure 2
Cumulative incidence rate (IR) (per 10,000 persons) of the newly diagnosed vaccination-related major events of interest. (A) Myocarditis. (B) Myocardial infarction. (C) Atrial fibrillation. (D) Interstitial lung disease. (E) Pulmonary thromboembolism. (F) Deep vein thrombosis. (G) Cerebrovascular disease. *p < 0.05; **p < 0.01; ***p < 0.001.
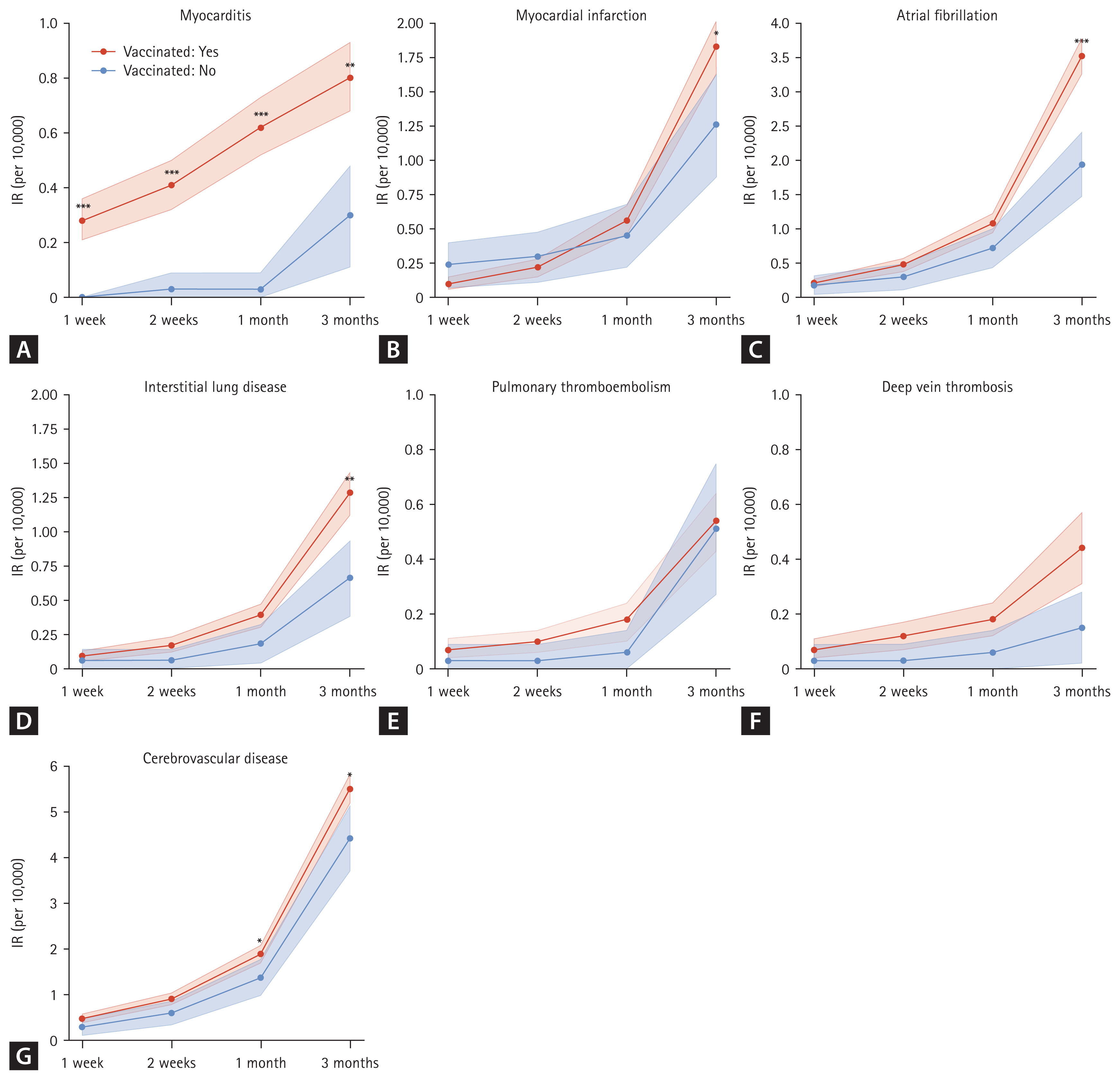
Figure 3
Forest plot of the adjusted logistic regression model in newly diagnosed patients with vaccination-related major events. aOR, adjusted odds ratio; CI, confidence interval; MI, myocardial infarction; AF, atrial fibrillation; ILD, interstitial lung disease; PTE, pulmonary thromboembolism; DVT, deep vein thrombosis; CeVD, cerebrovascular disease.
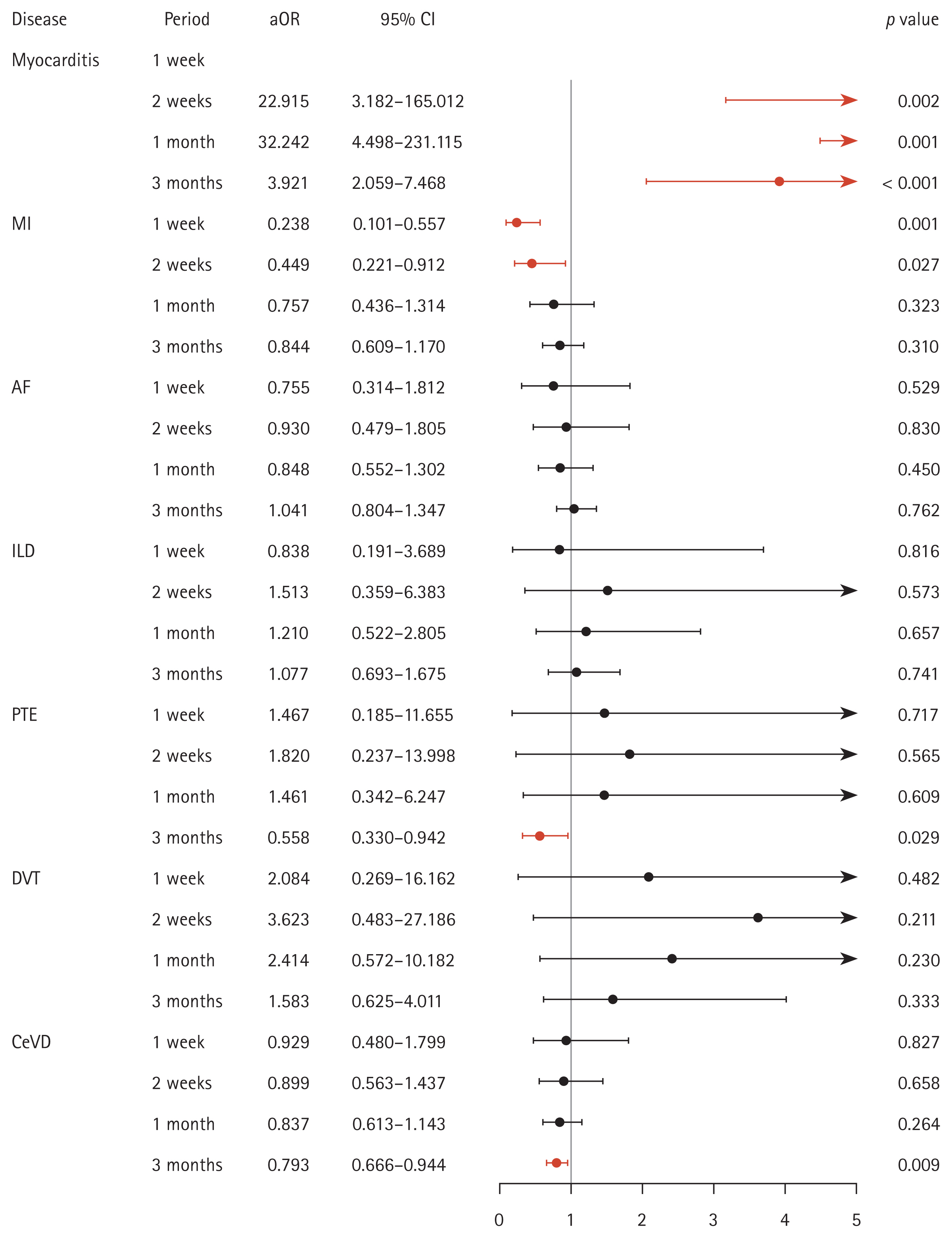
Figure 4
Forest plot of the adjusted Cox analysis model in newly diagnosed patients with vaccination-related major events. aHR, adjusted hazard ratio; CI, confidence interval; MI, myocardial infarction; AF, atrial fibrillation; ILD, interstitial lung disease; PTE, pulmonary thromboembolism; DVT, deep vein thrombosis; CeVD, cerebrovascular disease.
Table 1
Demographic characteristics of the study population according to vaccination status
REFERENCES
1. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021;6:104.




2. Polack FP, Thomas SJ, Kitchin N, et al.; 4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615.

3. Falsey AR, Sobieszczyk ME, Hirsch I, et al.; AstraZeneca AZD1222 Clinical Study Group. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med 2021;385:2348–2360.

4. Baden LR, El Sahly HM, Essink B, et al.; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–416.

5. Sadoff J, Gray G, Vandebosch A, et al.; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384:2187–2201.


6. Heath PT, Galiza EP, Baxter DN, et al.; 2019nCoV-302 Study Group. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 2021;385:1172–1183.

7. Food and Drug Administration. Emergency use authorization for vaccines to prevent COVID-19 [Internet] Silver Spring Food and Drug Administration, c2022. [cited 2023, 26 Aug]. Available from: https://downloads.regulations.gov/FDA-2020-D-1137-0019/attachment_1.pdf.
8. Thomas SJ, Moreira ED Jr, Kitchin N, et al.; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 2021;385:1761–1773.

9. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med 2021;385:2132–2139.


10. Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med 2021;385:1680–1689.


11. McKean N, Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep 2021;14:e244125.



12. Li X, Ostropolets A, Makadia R, et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ 2021;373:n1435.


13. Willame C, Dodd C, Durán CE, et al. Background rates of 41 adverse events of special interest for COVID-19 vaccines in 10 European healthcare databases - an ACCESS cohort study. Vaccine 2023;41:251–262.


14. Guo W, Deguise J, Tian Y, et al. Profiling COVID-19 vaccine adverse events by statistical and ontological analysis of VAERS case reports. Front Pharmacol 2022;13:870599.



15. Kim HJ, Kim MH, Park SJ, Choi MG, Chun EM. Autoimmune adverse event following COVID-19 vaccination in Seoul, South Korea. J Allergy Clin Immunol 2024;153:1711–1720.


16. Kim HJ, Kim MH, Choi MG, Chun EM. Psychiatric adverse events following COVID-19 vaccination: a population-based cohort study in Seoul, South Korea. Mol Psychiatry 2024;29:3635–3643.




17. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288–1294.


18. Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and pericarditis following mRNA COVID-19 vaccination: what do we know so far? Children (Basel) 2021;8:607.



19. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022;327:331–340.


20. Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ 2021;375:e068665.


21. Gao J, Feng L, Li Y, et al. A systematic review and meta-analysis of the association between SARS-CoV-2 vaccination and myocarditis or pericarditis. Am J Prev Med 2023;64:275–284.


22. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol 2022;19:75–77.



23. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484.



24. Mulroney TE, Pöyry T, Yam-Puc JC, et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2024;625:189–194.



25. Wong HL, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet 2022;399:2191–2199.



26. Cho JY, Kim KH, Lee N, et al. COVID-19 vaccination-related myocarditis: a Korean nationwide study. Eur Heart J 2023;44:2234–2243.




27. Andrews NJ, Stowe J, Ramsay ME, Miller E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: a national cohort study in England. Lancet Reg Health Eur 2022;13:100260.


28. Bikdeli B, Jiménez D, Demelo-Rodriguez P, et al. Venous thrombosis within 30 days after vaccination against SARS-CoV-2 in a multinational venous thromboembolism registry. Viruses 2022;14:178.



29. Smadja DM, Yue QY, Chocron R, Sanchez O, Lillo-Le Louet A. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J 2021;58:2100956.



30. Cascio Rizzo A, Giussani G, Agostoni EC. Ischemic stroke and vaccine-induced immune thrombotic thrombocytopenia following COVID-19 vaccine: a case report with systematic review of the literature. Cerebrovasc Dis 2022;51:722–734.



31. Abrignani MG, Murrone A, De Luca L, et al. COVID-19, vaccines, and thrombotic events: a narrative review. J Clin Med 2022;11:948.



32. Tsilingiris D, Vallianou NG, Karampela I, Dalamaga M. Vaccine induced thrombotic thrombocytopenia: the shady chapter of a success story. Metabol Open 2021;11:100101.



33. Hippisley-Cox J, Patone M, Mei XW, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 2021;374:n1931.


34. Chui CSL, Fan M, Wan EYF, et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: a self-controlled case series study. EClinicalMedicine 2022;50:101504.



35. Houghton DE, Wysokinski W, Casanegra AI, et al. Risk of venous thromboembolism after COVID-19 vaccination. J Thromb Haemost 2022;20:1638–1644.




36. Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021;27:1290–1297.




37. Kim YE, Huh K, Park YJ, Peck KR, Jung J. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA 2022;328:887–889.



38. Zisis SN, Durieux JC, Mouchati C, Perez JA, McComsey GA. The protective effect of coronavirus disease 2019 (COVID-19) vaccination on postacute sequelae of COVID-19: a multi-center study from a large national health research network. Open Forum Infect Dis 2022;9:ofac228.




39. Helms J, Tacquard C, Severac F, et al.; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–1098.




40. Burn E, Duarte-Salles T, Fernandez-Bertolin S, et al. Venous or arterial thrombosis and deaths among COVID-19 cases: a European network cohort study. Lancet Infect Dis 2022;22:1142–1152.



41. Xie J, Prats-Uribe A, Feng Q, et al. Clinical and genetic risk factors for acute incident venous thromboembolism in ambulatory patients with COVID-19. JAMA Intern Med 2022;182:1063–1070.



42. Nikolai LA, Meyer CG, Kremsner PG, Velavan TP. Asymptomatic SARS Coronavirus 2 infection: invisible yet invincible. Int J Infect Dis 2020;100:112–116.



43. Yoo H, Kim SY, Park MS, et al. COVID-19 vaccine-associated pneumonitis in the Republic of Korea: a nationwide multi-center survey. J Korean Med Sci 2023;38:e106.




44. So C, Izumi S, Ishida A, et al. COVID-19 mRNA vaccine-related interstitial lung disease: two case reports and literature review. Respirol Case Rep 2022;10:e0938.


45. Abutaleb MH, Makeen HA, Meraya AM, et al. Risks of cardiac arrhythmia associated with COVID-19 vaccination: a systematic review and meta-analysis. Vaccines (Basel) 2023;11:112.



46. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022;28:410–422.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print



