 |
 |
| Korean J Intern Med > Volume 40(6); 2025 > Article |
|
Abstract
Background/Aims
Cardiovascular-kidney-metabolic (CKM) syndrome is a continuum of metabolic, cardiovascular, and kidney dysfunctions. This study aimed to evaluate the association between CKM stages and the risk of adverse composite clinical outcomes.
Methods
This retrospective cohort study used data from the Korean National Health Insurance Database and included 1,497,913 individuals who underwent at least two health checkups between 2009 and 2012. The participants were classified into CKM stages (0–4), and the primary outcome was a composite of all-cause death, myocardial infarction, ischemic stroke, hemorrhagic stroke, and hospitalization for heart failure.
Results
The distribution of CKM stages was 17.4% (stage 0), 15.7% (stage 1), 57.6% (stage 2), 6.3% (stage 3), and 3.1% (stage 4). The incidence rate of primary outcomes increased progressively across the CKM stages, from 2.07 per 1,000 person-years in stage 0 to 40.70 per 1,000 person-years in stage 4. Compared with stage 0, the adjusted hazard ratios (HRs) for the primary outcome were significantly elevated: stage 1 (HR 1.09; 95% confidence interval [CI] 1.06–1.13; p < 0.001), stage 2 (HR 1.36; 95% CI 1.32–1.39; p < 0.001), stage 3 (HR 1.72; 95% CI 1.67–1.77; p < 0.001), and stage 4 (HR 2.70; 95% CI 2.62–2.79; p < 0.001).
Conclusions
A higher CKM stage was associated with a progressive increase in the risk of all-cause mortality and major cardiovascular events. Clinicians may benefit from prioritizing the early identification of high-risk individuals and implementing targeted management strategies based on CKM staging to improve long-term adverse outcomes.
Cardiovascular-kidney-metabolic (CKM) syndrome has recently emerged as a comprehensive framework for understanding the continuum of metabolic, cardiovascular, and kidney dysfunction, which collectively contribute to a significant proportion of global morbidity and mortality [1,2]. This conceptual framework reflects the growing recognition of the interconnectedness of these three systems and the need to consolidate them into clinical and research approaches. CKM syndrome has been proposed to address the limitations of previous compartmentalized definitions, such as metabolic syndrome, chronic kidney disease (CKD), and cardiovascular disease (CVD), which typically do not capture the synergistic effects of multi-system dysfunction [1–3]. The momentum for CKM syndrome stemmed from the need to better define the pathophysiological overlap and clinical importance of conditions such as obesity, type 2 diabetes, hypertension, CKD, and atherosclerotic CVD (ASCVD) [4–8]. The coexistence of these conditions accelerates disease progression and worsens outcomes beyond the sum of their individual effects [9–12]. To address this, by staging individuals based on metabolic, cardiovascular, and renal parameters, CKM staging allows for identifying high-risk populations who may benefit from tailored therapeutic approaches [1]. Additionally, incorporating CKM staging into population health strategies may improve long-term outcomes by managing shared risk factors and pathophysiological mechanisms.
Despite the importance of CKM staging, evidence on the influence of CKM stage on long-term clinical outcomes is limited, particularly in large, diverse populations. This knowledge gap hinders the development of effective management and prevention strategies that target CKM-related risks. Therefore, this study evaluated the association between CKM stages and the risk of adverse composite clinical outcomes, including all-cause mortality and major cardiovascular events.
Although the outcomes were followed prospectively from baseline, this retrospective cohort study used pre-existing administrative and screening data from the Korean National Health Insurance Database (NHID). The National Health Insurance program covers approximately 97% of the Korean population; moreover, a majority of this population access medical services at least once a year. The NHID, as previously described [13,14], uses the International Classification of Diseases, Tenth Revision (ICD-10) codes to document diagnoses. We included individuals aged ≥ 20 years who had completed at least two health checkups between 2009 and 2012 (dataset number NHIS-2024-1-258) using stratified random sampling to improve the completeness and reliability of baseline covariate data. The initial sample consisted of 1,500,959 individuals; however, after excluding those aged ≥ 90 years and those with missing or biologically implausible health checkup data for CKM staging or covariates (n = 3,046, 0.2%), a total of 1,497,913 participants were included. Participants were categorized into five CKM stages for subsequent analyses.
This study was approved by the institutional review board of Hanyang University Guri Hospital (approval no. GURI 2024-12-021). The requirement for informed consent was waived as the NHID had obtained the participants’ consent. The dataset is publicly available and contains no individually identifiable information.
Key variables included demographic details such as age and sex at the initial health checkup. Blood pressure (BP) measurements encompassed systolic BP and diastolic BP. These measurements were obtained during the National Health Insurance Service (NHIS) health screening examination using standardized procedures, including seated rest for at least 5 minutes before measurement with automated devices. The average of two readings was recorded when available. All participating centers followed uniform protocols under NHIS supervision. Smoking status was categorized as never, past, or current smoker. Physical activity levels were documented based on frequency and grouped into 0, 1–2, 3–4, 5–6, or 7 sessions per week. Alcohol consumption was categorized as 0, 1–2, 3–4, or ≥ 5 times per week. Body mass index (BMI) was stratified as < 18.5, 18.5–22.9, 23–24.9, and ≥ 25 kg/ m2. Fasting glucose levels were categorized as < 100, 100– 125.9, or ≥ 126 mg/dL, while total cholesterol levels were grouped as < 200, 200–239.9, or ≥ 240 mg/dL. Low-density lipoprotein cholesterol levels were assessed, and high-density lipoprotein cholesterol levels were categorized as < 40 mg/dL in men and < 50 mg/dL in women. Triglyceride levels ≥ 150 mg/dL were also evaluated. Household income was divided into quartiles, with the lowest and highest income groups representing the first and fourth quartiles, respectively. To represent household income, the average monthly insurance premium determined by government assessments of salaries and assets was used. Health insurance premiums are assessed proportionally based on salary for workplace health insurance subscribers and income or property for local health insurance subscribers in South Korea [14,15]. Medical history included diagnoses of hypertension, diabetes, and dyslipidemia. The use of antihypertensive medications (diuretics, beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, etc.) was documented, and the number of agents was categorized as 1, 2, 3, or ≥ 4. Data on glucose- and lipid-lowering medications and antiplatelet therapies were also recorded. The PREVENT score [16] was used to obtain a comprehensive risk profile for participants in stages 0 to 3 only, as it is not validated for individuals with established CVD (stage 4). Thus, PREVENT scores were not calculated or reported for stage 4 participants.
CKM syndrome stages were defined using detailed clinical criteria (Supplementary Table 1), incorporating metabolic dysfunction, cardiovascular risk factors, and kidney function: stage 0, individuals with normal BMI, waist circumference, fasting glucose levels, lipid profile, and BP, with no evidence of CKD or subclinical/clinical CVD; stage 1, individuals who were overweight, with abdominal obesity, or dysfunctional adiposity without additional metabolic risk factors or CKD (criteria included BMI ≥ 23 kg/m2, waist circumference ≥ 85 cm for women or ≥ 90 cm for men, or fasting glucose between 100 and 125 mg/dL [17]; stage 2, presence of metabolic risk factors, such as hypertriglyceridemia (≥ 135 mg/dL, as recently defined by the American Heart Association (AHA) CKM staging framework [1]), hypertension, metabolic syndrome, diabetes, or CKD (estimated glomerular filtration rate 30–60 mL/min/1.73 m2, based on Kidney Disease: Improving Global Outcomes guidelines [18]); stage 3, evidence of subclinical ASCVD or subclinical heart failure (HF) among individuals with CKD or metabolic risk factors; and stage 4, clinical CVD, including coronary artery disease (CAD), HF, stroke, peripheral artery disease (PAD), or atrial fibrillation (AF) among individuals with excess/dysfunctional adiposity, other CKM risk factors, or CKD. The BMI and waist circumference criteria were based on recommendations tailored for Asian populations by World Health Organization Western Pacific Regional Office and Korean Society for the Study of Obesity [19,20]. These stages were developed to capture a continuum of CKM progression, highlighting the integration of metabolic, cardiovascular, and renal factors. Further details on differences between our CKM staging criteria and the original AHA framework are summarized in Supplementary Table 2.
The primary outcome was a combination of all-cause mortality, myocardial infarction (MI), ischemic stroke, hemorrhagic stroke, and hospitalization for HF during the follow-up period, which was extended until December 31, 2022. The mean follow-up duration was 12.60 ± 1.50 years, with a median of 13.02 years (interquartile range: Q1 12.25 years, Q3 13.39 years). Secondary outcomes included the individual components of the primary composite outcome. Additional cardiovascular outcomes, such as CAD, PAD, AF, and hospitalization for AF, were analyzed individually. Mortality data including the date of death were obtained from the National Statistical Office of Korea. Diagnoses and procedural definitions, including those for MI, HF, ischemic stroke, hemorrhagic stroke, CAD, PAD, and AF, were based on the ICD-10 and procedural codes detailed in Supplementary Table 3. Because of the universal coverage and centralized claims system of the NHID, loss to follow-up was negligible, and censoring was primarily due to death or reaching the end of the observation period.
Baseline characteristics across CKM stages were compared using chi-square tests for categorical variables and analysis of variance for continuous variables. Incidence rates were determined as the total number of events divided by the cumulative person-years (PY) of follow-up, expressed as per 1,000 PY. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the composite outcome and its components across CKM stages, using stage 0 as the reference group. The models were adjusted as follows: Model 1 included age and sex; Model 2 included age, sex, smoking status, alcohol consumption, physical activity, and household income; Model 3 was further adjusted for the use of antihypertensive, glucose-lowering, lipid-lowering, and antiplatelet agents. Kaplan–Meier survival curves were constructed to visualize event-free survival according to the CKM stage, and differences were tested using the log-rank test. Cox proportional hazard models were used to generate directly adjusted survival curves [21–23] based on Model 3 by averaging the estimated survival curves. We assessed the proportional hazards assumption using Schoenfeld residual tests and log-minus-log survival curves to evaluate the validity of the Cox proportional hazards models. Statistical significance was set at a two-tailed p value < 0.05. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Baseline characteristics of the study population stratified by CKM stage are presented in Table 1 and Supplementary Table 4. Among the 1,497,913 participants, the distribution across CKM stages revealed that stage 2 accounted for the largest proportion (57.5%), followed by stages 0 (17.4%), 1 (15.7%), 3 (6.3%), and 4 (3.1%). Significant differences were observed across the CKM stages for several variables, including age, sex, smoking status, physical activity, alcohol consumption, BMI, fasting glucose levels, lipid profiles, and comorbidities. As expected from the CKM staging definitions, participants in the higher stages, particularly stages 3 and 4, were older and exhibited more adverse metabolic and cardiovascular profiles, including higher BP, fasting glucose, and triglyceride levels, as well as a greater prevalence of hypertension, diabetes, and dyslipidemia. Additionally, the use of antihypertensive, glucose-lowering, and lipid-lowering medications significantly increased with CKM stage progression (p < 0.001). Although these differences were statistically significant, they highlight the progressive accumulation of risk factors and comorbidities, particularly in more advanced CKM stages, underscoring the need for the targeted management of these high-risk groups.
Incidence rates of the composite primary outcomes per 1,000 PY across different CKM stages are presented in Table 2. The incidence rate increased progressively from 2.07 per 1,000 PY in stage 0 to 40.70 per 1,000 PY in stage 4. With stage 0 as the reference category, higher CKM stages were associated with a significant increase in the risk of composite outcomes. Particularly, stage 1 had an adjusted HR of 1.09 (95% CI 1.06–1.13; p < 0.001), and the risk progressively increased in stages 2 (HR 1.36; 95% CI 1.32–1.39; p < 0.001), 3 (HR 1.72; 95% CI 1.67–1.77; p < 0.001), and 4 (HR 2.70; 95% CI 2.61–2.79; p < 0.001). These results were consistent even after adjusting for potential confounders, including age, sex, smoking status, alcohol consumption, physical activity, household income, and antihypertensive, glucose-lowering, lipid-lowering, and antiplatelet medications. The incidence rates for all secondary outcomes, including all-cause death, MI, ischemic stroke, hemorrhagic stroke, and hospitalization for HF, CAD, PAD, and AF, followed a similar trend. Higher CKM stages consistently demonstrated poor outcomes, with the adjusted HRs remaining significant across all secondary outcomes after controlling for the same confounding variables. Figure 1 presents the directly adjusted event curves generated using Cox proportional hazards models based on Model 3, averaging the estimated survival curves. These curves visually confirm a progressive increase in the cumulative incidence of events with higher CKM stages. Stage 4 participants consistently exhibited the highest event rates for all measured endpoints, including the composite primary outcome, all-cause death, and major cardiovascular events.
To address concerns regarding the validated age range of the PREVENT equations, we conducted a sensitivity analysis restricted to participants aged 30–79 years. The distribution of CKM stages and associated risk trends remained consistent with the main analysis (Supplementary Table 5). Additionally, to address potential bias from combining primary and secondary prevention populations, we performed a subgroup analysis restricted to primary-prevention individuals (stages 0–3), which revealed a similar progressive increase in the risk of adverse outcomes across CKM stages, supporting our CKM staging system (Supplementary Table 6). Furthermore, subgroup analyses in CKM stage 4 revealed that patients with HF (HR 1.48; 95% CI 1.43–1.54) or PAD (HR 1.31; 95% CI 1.15–1.48) faced a higher risk for the composite primary outcome. In contrast, patients with CAD had a lower adjusted risk (HR 0.89; 95% CI 0.86–0.93) than patients without CAD in stage 4 (Supplementary Table 7). These findings highlight the different prognostic impacts of specific CVDs in stage 4.
Supplementary Figure 1 shows the Kaplan–Meier curves for each outcome stratified by the CKM stage. The curves distinctly separated the event rates across the CKM stages for all endpoints, including the composite primary outcome, all-cause death, and hospitalization for HF, stroke, and MI. For the composite primary outcome (Supplementary Fig. 1A), stages 3 and 4 crossed during the follow-up period. For all-cause death (Supplementary Fig. 1B), stage 3 exhibited higher event rates than stage 4. These discrepancies highlight the limitations of Kaplan–Meier curves, which do not account for covariate adjustments. In contrast, the adjusted event curves in Figure 1, derived from Model 3, accounted for potential confounders and provided a more robust analysis. Despite these differences, both curves demonstrated a progressive increase in adverse outcomes with advancing CKM stages. The global Schoenfeld test yielded a statistically significant p value (< 0.001), likely influenced by the large sample size. However, visual inspection of the Schoenfeld residual plot revealed no noticeable deviation from randomness over time, indicating that the proportional hazards assumption was not meaningfully violated (Supplementary Fig. 2). The log-minus-log survival curves also demonstrated approximate parallelism across CKM stages, further supporting the validity of the Cox models (Supplementary Fig. 3).
The findings of this study highlight the significant effects of CKM progression on long-term clinical outcomes in a large nationwide population-based cohort. The CKM stage was strongly associated with a progressive increase in the risk of adverse outcomes, including composite primary outcomes, all-cause death, and major cardiovascular events, despite adjusting for key confounding factors. Notably, participants in stage 1 or 2 demonstrated an increased risk of adverse outcomes compared to those in stage 0, underlining the clinical significance of early-stage metabolic dysfunction and risk factor clustering. The progressive increase in risk across CKM stages highlights the combined effects of metabolic dysfunction, cardiovascular risk factors, and kidney impairment on adverse outcomes. These results suggest that early identification and targeted interventions in individuals with higher CKM stages might be important to potentially mitigate long-term clinical risks. These findings are particularly relevant given that East Asian populations exhibit distinct ASCVD risk profiles, mortality patterns, and metabolic characteristics compared to Western populations, as highlighted by Nguyen et al. in their multinational analysis of ASCVD risk models in China, Japan, and Korea [24].
CKM syndrome highlights the interconnected and bidirectional associations between the metabolic, cardiovascular, and kidney systems. This framework determines the combined effects of metabolic dysfunction, renal impairment, and CVD, which collectively aggravate disease progression and cause poor clinical outcomes. Pathophysiologically, CKM syndrome involves a cascade of systemic inflammation, oxidative stress, endothelial dysfunction, and neurohormonal activation, intensifying the risk of adverse events [1,25,26]. Obesity and insulin resistance, key drivers of CKM syndrome, trigger chronic low-grade inflammation by releasing pro-inflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, from adipose tissue. These cytokines disrupt endothelial function, promote atherosclerosis, and accelerate kidney injury [26,27]. Similarly, CKD contributes to cardiovascular risk through mechanisms such as uremic toxin accumulation, vascular calcification, and renin-angiotensin-aldosterone system activation, which exacerbate hypertension and left ventricular hypertrophy [25,28]. Similarly, metabolic dysfunction leads to dyslipidemia and hyperglycemia, causing vascular injury and glomerular hyperfiltration, thus creating a vicious cycle [27,28]. The clinical implications of CKM syndrome are substantial. Individuals with coexisting conditions, such as diabetes and CKD, experience a synergistic increase in cardiovascular mortality compared with those with isolated conditions [25,29]. CKM staging provides a structured method for stratifying risk by combining metabolic, renal, and cardiovascular parameters. This staging allows for the early identification of high-risk individuals and the performance of targeted interventions to mitigate long-term complications [1,29]. Furthermore, CKM syndrome focusses the need for multidisciplinary care models that manage shared risk factors, such as obesity, dyslipidemia, and hypertension, across these complementary systems [1,6].
The findings of this study align with those of previous studies, highlighting the progressive nature of CKM syndrome and its significant impact on clinical outcomes. Numerous studies have demonstrated the interplay between metabolic dysfunction, CVD, and CKD; however, few have systematically quantified risk progression across clearly defined CKM stages. By providing a comprehensive risk assessment in a large population-based cohort, this study fills critical gaps in the literature [1,3,6,25]. While stage 4 includes individuals with established CVD and thus reflects the risk of recurrent events rather than incident events, we included this group to illustrate the continuum of cardiovascular and metabolic risk as defined in the CKM classification. To address the potential bias raised by comparing primary and secondary prevention groups, we also performed a subgroup analysis restricted to stages 0 to 3, which is provided in Supplementary Table 6. Importantly, this subgroup analysis demonstrated a consistent stepwise increase in clinical risk across CKM stages, similar to the main analysis. Minhas et al. [29] and Aggarwal et al. [2] have highlighted the prevalence and public health imports of CKM stages in the US population; however, their studies primarily focused on descriptive epidemiology rather than outcome-specific stratification. Our study develops this understanding by quantifying the progressive increase in the risk of composite and secondary outcomes across the CKM stages. Similarly, the results of the Kailuan study by Li et al. [9] demonstrated that CKM staging is a strong predictor of all-cause mortality in the Chinese population, with stage 4 showing an HR of 3.73 compared to stage 0. Unlike the Kailuan Study, which focused only on all-cause mortality, our study investigated a broader scope of outcomes, including major cardiovascular events such as MI, ischemic stroke, hemorrhagic stroke, and hospitalization for HF. This comprehensive approach allows for a more detailed understanding of the influence of CKM stage on diverse clinical endpoints, underscoring the broader clinical implications of CKM staging in risk stratification and guiding targeted interventions. In a subgroup analysis within stage 4, the adjusted HR was increased among patients with HF, stroke, or PAD. Interestingly, patients with established CAD exhibited a lower adjusted HR compared to those without CAD. This unexpected finding may reflect the implementation of intensive secondary preventive interventions, including optimal medical therapies and lifestyle modifications that are routinely applied following a diagnosis of CAD. Such treatment effects may attenuate their relative risk compared to other cardiovascular subgroups within the same CKM stage. However, this hypothesis should be examined further in prospective studies.
Our study complements the research on therapeutic interventions by emphasizing the need for stage-specific management strategies for CKM syndrome. Herrington et al. [30] demonstrated the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in reducing cardiovascular and renal outcomes in patients with CKD, while Bethel et al. [31] highlighted the benefits of glucagon-like peptide-1 (GLP-1) receptor agonists in reducing major adverse cardiovascular events in patients with diabetes. However, these studies lacked an integrated framework to capture the combined effects of CKM-related conditions. Considering the progressive increase in risk across CKM stages observed in our study, we recommend using the CKM staging framework may serve as a useful reference for considering treatments such as GLP-1 receptor agonists and SGLT2 inhibitors in clinical practice. Specifically, stage 2 may act as an early signal for clinicians to proactively initiate intensive lifestyle modifications, such as dietary interventions, increased physical activity, and weight management programs. Stage 3 may justify initiation of pharmacologic therapies, including SGLT2 inhibitors and statins, given the substantial cardiovascular and renal protective effect. However, due to the observational and retrospective nature of our study, direct evidence supporting these specific recommendations cannot be provided. Additionally, it is important to note that SGLT2 inhibitors were not yet approved in Korea during the baseline period of this study (2009–2012), and most GLP-1 receptor agonists became available only in later years. As a result, the actual use of these agents in our cohort was extremely limited and did not influence the outcome estimates. Nonetheless, the staging framework may serve as a useful clinical tool to guide the application of these therapies in future prospective studies and real-world settings.
Despite its strengths, this study had several limitations. First, the CKM staging was adjusted because of the limitations of the available data from the NHID. In particular, the stage 3 criteria for subclinical ASCVD and subclinical HF could not be fully captured, as the database lacked information on coronary angiography, cardiac imaging (e.g., echocardiography), and biomarkers such as troponin and B-type natriuretic peptides. As the dataset was based on health checkup claims data, asymptomatic individuals with undiagnosed ASCVD or HF may not have been identified. This limitation affects the ability to detect early stages of the disease, and asymptomatic individuals with undetected subclinical diseases may have been misclassified into lower stages, potentially underestimating the prevalence of advanced CKM stages and attenuating the observed associations between CKM stages and clinical outcomes. Although our CKM stage definitions were adapted from the AHA framework, some modifications were necessary due to the inherent limitations of the NHID-based data. These transformations, while pragmatically justified, may introduce minor classification differences and should be interpreted accordingly. Second, reliance on claims data introduces inherent limitations, such as variability in diagnostic coding practices and potential misclassification of certain comorbidities or outcomes. Although strict exclusion criteria were applied to ensure data quality, coding inaccuracies remained a possible source of bias. However, the large sample size of this study, comprising nearly 1.5 million individuals, possibly mitigated the impact of such inaccuracies by enhancing the statistical power and robustness of the findings. Third, although the study accounted for numerous confounding variables in the analysis, some relevant factors could not be included due to data limitations. Variables such as dietary patterns, physical activity intensity, genetic predispositions, family history of CVD, CKD, or diabetes, and sleep health were not captured in the dataset. Although we used household income to represent socioeconomic status, other social determinants of health were not available. In particular, variables included in the Social Deprivation Index—such as educational attainment, household structure (e.g., single-parent households), and access to transportation—were not available in the NHID. This may have limited our ability to capture multidimensional social vulnerability. These unmeasured variables may have contributed to residual confounding in our findings. Fourth, because our dataset was not linked to the national cause-of-death registry, we were unable to analyze cause-specific mortality, such as cardiovascular, cancer, or infection-related death. Thus, our analyses were limited to all-cause mortality, which prevented competing risk analysis and potentially led to an incomplete understanding of cardiovascular-specific risks associated with CKM stages. Future studies should use datasets with detailed cause-specific mortality information to fully address competing risks and refine the cardiovascular risk predictions in CKM populations. Finally, as this study focused on a large Asian population, the findings may not be directly generalizable to other racial or ethnic groups owing to potential genetic, lifestyle, and healthcare differences. Further research, including various populations, is required to analyze racial and regional variations in CKM syndrome and validate the applicability of these findings globally.
CKM syndrome provides a comprehensive framework for managing the interconnected risks of metabolic, cardiovascular, and kidney dysfunctions, emphasizing the importance of combined stage-specific management strategies. Although CKM staging may not capture all aspects of these complex conditions, it provides a useful risk stratification and integrated management tool. By systematically quantifying the progressive increase in risk across the CKM stages, this study highlights its potential utility in clinical practice and health policy as a more holistic approach than managing metabolic syndrome, CKD, or CVD in isolation. Further research is needed to refine the CKM staging and validate its role in improving patient outcomes and guiding targeted interventions.
Notes
Acknowledgments
This study used data from the National Health Insurance Service database (NHIS-2024-1-258).
CRedit authorship contributions
Hyun-Jin Kim: methodology, investigation, formal analysis, software, writing - original draft, writing - review & editing; Byung Sik Kim: methodology, investigation, formal analysis, software, writing - original draft, writing - review & editing; Hasung Kim: methodology, investigation, data curation, formal analysis; Jungkuk Lee: methodology, investigation, data curation, formal analysis; Ha Hye Jo: methodology, investigation; Dong Wook Kim: methodology, writing - review & editing; Jeong-Hun Shin: conceptualization, supervision, writing - review & editing; Ki-Chul Sung: investigation, writing - review & editing
Figure 1
Adjusted event curves across CKM stages. Directly adjusted event curves were generated using Cox proportional hazards models based on Model 3, averaging the estimated survival curves. These curves illustrate the cumulative incidence of events across the CKM stages. (A) Composite primary outcome: The cumulative incidence of the composite outcome increased progressively, with stage 4 showing the highest event rate. (B) All-cause death: The cumulative incidence of death increased steeply with advancing CKM stages, with stage 4 participants experiencing the highest rates. (C) Hospitalization for heart failure: A significant increase in the cumulative incidence of hospitalization was evident, particularly in stages 3 and 4. (D) Stroke (ischemic or hemorrhagic): The cumulative incidence of stroke progressively increased with higher CKM stages, with stage 4 participants displaying the most significant risk. (E) Myocardial infarction: The cumulative incidence of myocardial infarction increased sharply in higher CKM stages, with stage 4 showing the poorest outcomes. CKM, cardiovascular-kidney-metabolic.
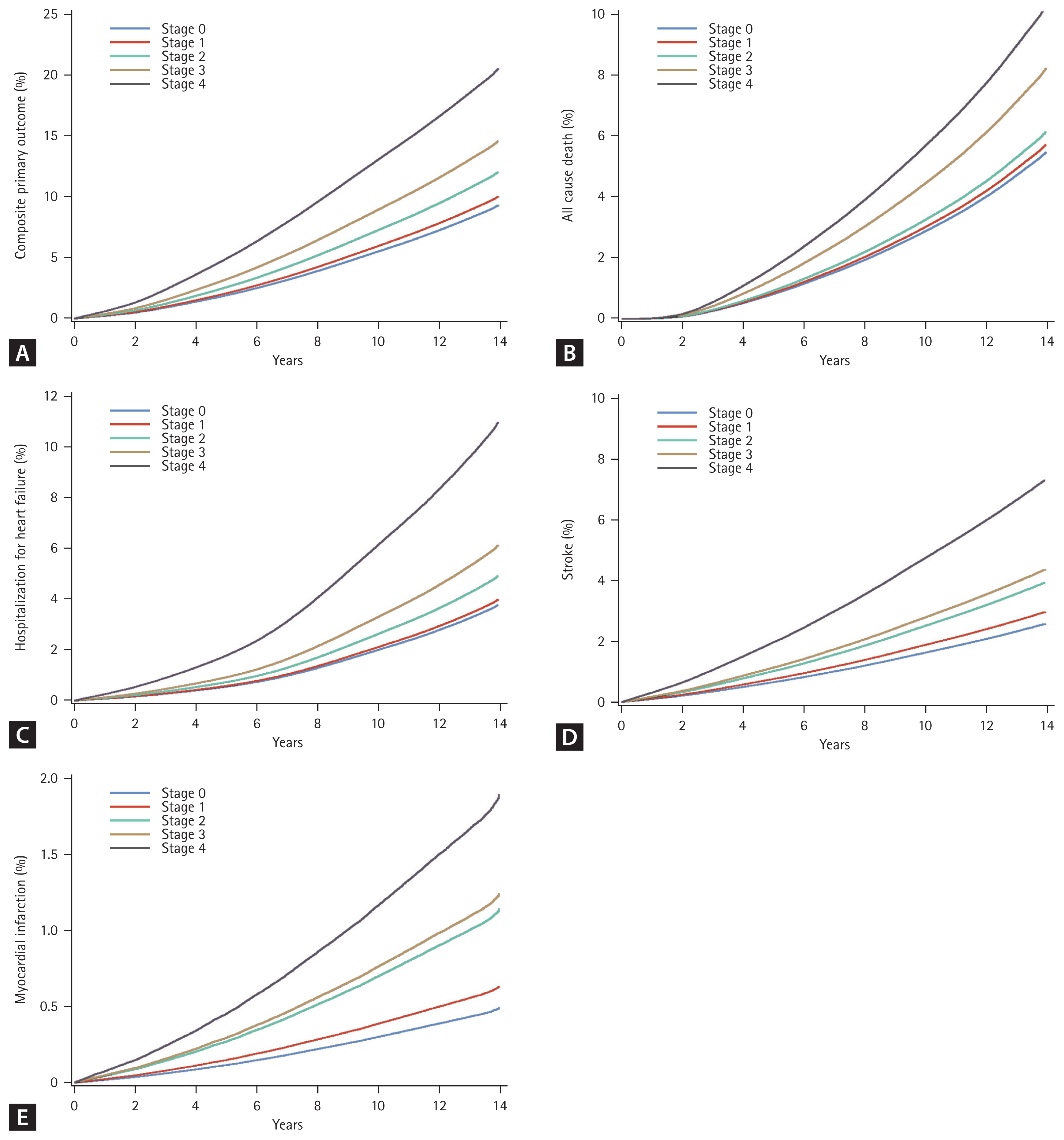
Table 1
Baseline characteristics
Table 2
Clinical outcomes according to CKM stage
AF, atrial fibrillation; CI, confidence interval; CKM, cardiovascular-kidney-metabolic; HF, heart failure; HR, hazard ratio; IR, incidence rate; Ref., reference.
Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, and household income. Model 3 was adjusted for age, sex, smoking status, alcohol consumption, physical activity, household income, use of antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs, and antiplatelet agents.
REFERENCES
1. Ndumele CE, Rangaswami J, Chow SL, et al.; American Heart Association. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation 2023;148:1606–1635.


2. Aggarwal R, Ostrominski JW, Vaduganathan M. Prevalence of cardiovascular-kidney-metabolic syndrome stages in US adults, 2011–2020. JAMA 2024;331:1858–1860.



3. Ndumele CE, Neeland IJ, Tuttle KR, et al.; American Heart Association. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation 2023;148:1636–1664.

5. Rangaswami J, Bhalla V, Blair JEA, et al.; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019;139:e840–e878.


6. Powell-Wiley TM, Poirier P, Burke LE, et al.; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021;143:e984–e1010.



7. Neeland IJ, Ross R, Després JP, et al.; International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7:715–725.


8. Joseph JJ, Deedwania P, Acharya T, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; and Council on Hypertension. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 2022;145:e722–e759.


9. Li N, Li Y, Cui L, et al. Association between different stages of cardiovascular-kidney-metabolic syndrome and the risk of all-cause mortality. Atherosclerosis 2024;397:118585.


10. Yen FS, Wei JC, Chiu LT, Hsu CC, Hwu CM. Diabetes, hypertension, and cardiovascular disease development. J Transl Med 2022;20:9.




11. Kishor S, Chen J, Zhang Y, et al. Interaction of proteinuria and diabetes on the risk of cardiovascular events: a prospective cohort CKD-ROUTE study. BMC Public Health 2024;24:3192.




12. Kim HJ, Kim BS, Lee Y, Ahn SB, Kim DW, Shin JH. Harnessing metabolic indices as a predictive tool for cardiovascular disease in a Korean population without known major cardiovascular event. Diabetes Metab J 2024;48:449–462.




13. Kim HJ, Lee HW, Kang MK, Leem GH, Kim MH, Song TJ. Association of body composition changes with the development of diabetes mellitus: a nation-wide population study. Diabetes Metab J 2024;48:1093–1104.




14. Shin JH, Jung MH, Kwon CH, et al. Disparities in mortality and cardiovascular events by income and blood pressure levels among patients with hypertension in South Korea. J Am Heart Assoc 2021;10:e018446.



15. Park EJ, Ji NJ, You CH, Lee WY. Healthcare utilization and discrepancies by income level among patients with newly diagnosed type 2 diabetes in Korea: an analysis of National Health Insurance Sample Cohort Data. J Prev Med Public Health 2024;57:471–479.




16. Khan SS, Matsushita K, Sang Y, et al.; Chronic Kidney Disease Prognosis Consortium and the American Heart Association Cardiovascular-Kidney-Metabolic Science Advisory Group. Development and validation of the American Heart Association’s PREVENT equations. Circulation 2024;149:430–449.



17. American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025;48:S27–S49.

18. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2024;105:S117–S314.


19. Kim KK, Haam JH, Kim BT, et al.; Committee of Clinical Practice Guidelines, Korean Society for the Study of Obesity (KSSO). Evaluation and treatment of obesity and its comorbidities: 2022 update of clinical practice guidelines for obesity by the Korean Society for the Study of Obesity. J Obes Metab Syndr 2023;32:1–24.



20. World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment [Internet] Sydney: Health Communications Australia, c2000. [cited 2025 June 22]. Available from: https://iris.who.int/handle/10665/206936.
22. Gail MH, Byar DP. Variance calculations for direct adjusted survival curves, with applications to testing for no treatment effect. Biom J 1986;28:587–599.

23. Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed 2007;88:95–101.


24. Nguyen PK, Zhao D, Okamura T, Chang Kim H, Wong ND, Yang E. Atherosclerotic cardiovascular disease risk prediction models in China, Japan, and Korea: implications for East Asians? JACC Asia 2025;5:333–349.



25. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021;143:1157–1172.



26. Castellon X, Bogdanova V. Chronic inflammatory diseases and endothelial dysfunction. Aging Dis 2016;7:81–89.



28. Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev 2019;40:1447–1467.




29. Minhas AMK, Mathew RO, Sperling LS, et al. Prevalence of the cardiovascular-kidney-metabolic syndrome in the United States. J Am Coll Cardiol 2024;83:1824–1826.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print



