 |
 |
| Korean J Intern Med > Volume 41(2); 2026 > Article |
|
Abstract
Notes
Figure 1
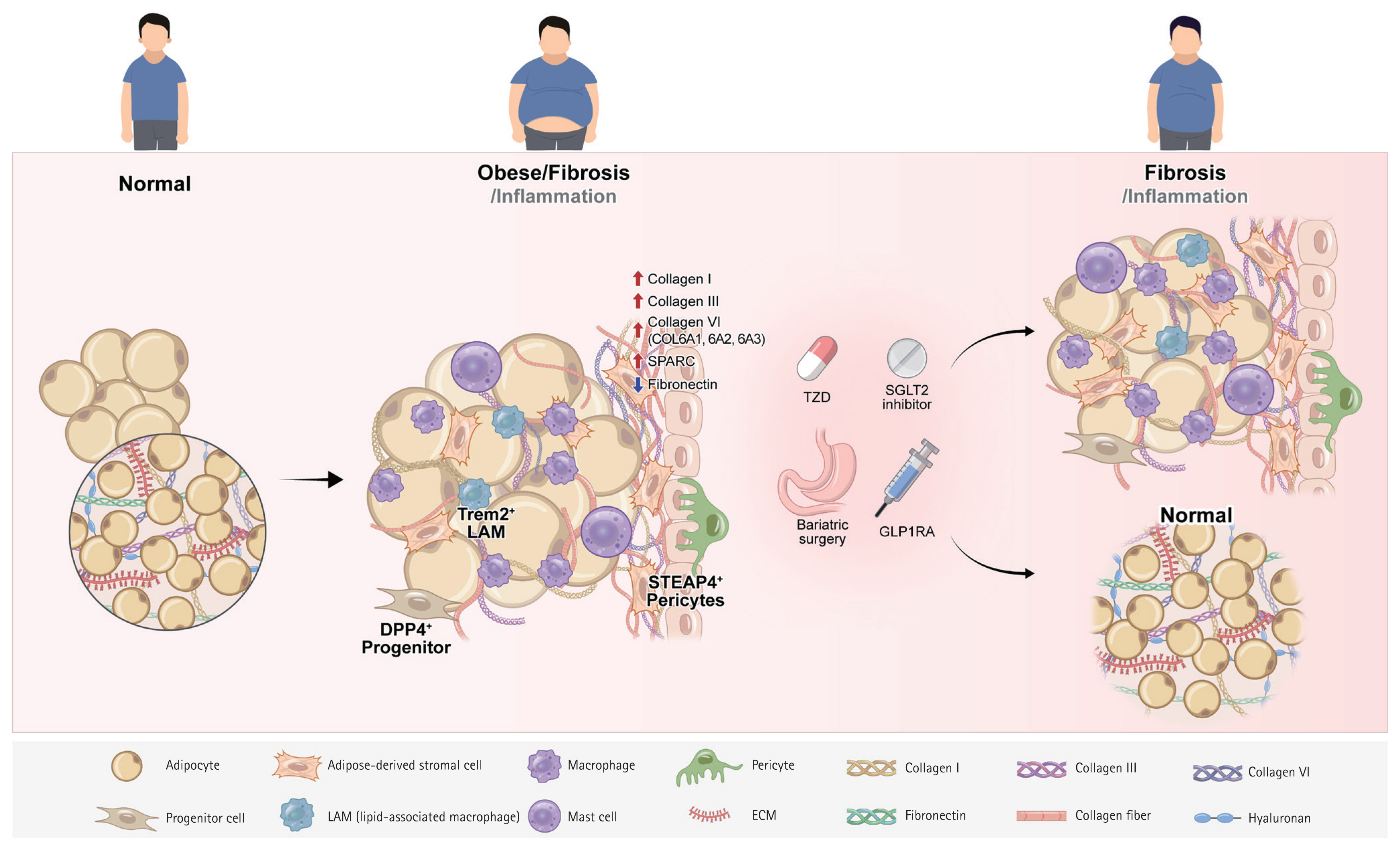
Table 1
| Study | Cell | Model | Depot/type | Marker | Key findings |
|---|---|---|---|---|---|
| Zhang et al. (2025) [26] | PDGFRα+ DPP4+ progenitors | Mouse | scAT | PDGFRα, DPP4, SMAD targets |
Switch: adipogenic → COL-producing fibroblast Expansion to ~60% of progenitors in fibrosis DPP4 inhibition: ↑ adipogenesis, ↓ COL deposition |
| Bailin et al. (2024) [33] | MYOC+ fibroblast | Human |
scAT CLS-adjacent zones |
ACTA2, COL1A1, COL6A1, MYOC |
↑ ECM gene expression Wnt/β-catenin & TGF-β activation Localized near hypoxic adipocytes Spatially organized ECM synthesis |
| Zhang et al. (2025) [30] | STEAP4+ pericytes | Human | Mesenteric WAT | STEAP4, PDGFRβ, COL3A1, FN1, LOX |
Transition: pericytes → matrix-producing myofibroblast-like cells Links microangiopathy with stromal fibrosis |
| Kohda et al. (2025) [28] | Mincle+ macrophages | Mouse | CLS in WAT | Clec4e (Mincle), OSM, TGF-β1 |
Potent fibroblast activation OSM: ↓ COL gene expression Loss of OSM → ↑ fibrosis Small (~2–3%) population with large effect |
| Reyes-Farias et al. (2025) [32] | CD45+CD11b+ Ly6a+ macrophages | Mouse | VAT | Collagen |
Pro-fibrotic but not pro-adipogenic CD45−CD11b−Ly6a+: Form lipid droplets & synthesize COL |
| Harasymowicz et al. (2021) [29] | CD9+TREM2high Lipid-associated macrophages | Mouse | VAT | TREM2, LPL, APOE |
Lipid handling & tissue remodeling during CLS formation Associated with metabolic stress adaptation |
| Liu et al. (2022) [27] | Adipocytes | Human/ mouse | WAT & BAT | COL1A1, COL3A1, COL4A1, COL6A3, CTGF, LOX, OPN |
Obesity: ↑ mature COL in AT AMPK activation: ↓ fibrosis & LOX expression in adipocytes |
| Hirai et al. (2014) [31] | Mast cells | Human/ mouse | scAT | Tryptase, chymase, MCP-6 |
↑ COL V & inhibit preadipocyte differentiation Linked to IR |
ACTA2, actin alpha 2, smooth muscle; AMPK, AMP-activated protein kinase; APOE, apolipoprotein E; AT, adipose tissue; BAT, brown adipose tissue; CLS, crown-like structure; COL, collagen; CTGF, connective tissue growth factor; DPP4, dipeptidyl peptidase-4; ECM, extracellular matrix; FN1, fibronectin 1; IR, insulin resistance; LOX, lysyl oxidase; LPL, lipoprotein lipase; MCP, monocyte chemoattractant protein; MYOC, myocilin; OPN, osteopontin; OSM, oncostatin M; PDGFRα, platelet-derived growth factor receptor alpha; PDGFRβ, platelet-derived growth factor receptor beta; scAT, subcutaneous adipose tissue; SMAD, mothers against decapentaplegic homolog; STEAP4, six-transmembrane epithelial antigen of prostate 4; TGF-β, transforming growth factor-beta; TREM2, triggering receptor expressed on myeloid cells 2; VAT, visceral adipose tissue; WAT, white adipose tissue; ↑, increase/increased/ higher; ↓, decrease/decreased/lower; ~, approximately.
Table 2
| Study | Model | Sample type | Treatment duration | Weight loss % | Fibrotic markers | Key findings |
|---|---|---|---|---|---|---|
| Cancello et al. 2013 [70] |
Human obese (n = 58) BS |
scAT | 18–24 mo post-op |
BW: 36% BMI: ≥ 40→27.2 |
Picrosirius Red Elevated ECM Persistent Col |
↓ Inflammation Persistent fibrosis Obesity gene pattern maintained |
| Kos et al. 2009 [77] |
Human obese (n = 24) VLCD |
scAT | 16 wk VLCD (450 kcal/d) + 2 wk refeeding | BW: 23.5% |
SPARC (mRNA/protein) ↓33% (wk 8) ↑20% (refeeding) |
SPARC ↓ with WL SPARC ↑ with WG Plateau after 8 wk |
| Abdennour et al. 2014 [64] | Human obese (n = 404; followed n = 243) |
scAT Omental AT Liver |
3, 6, 12 mo post-BS-op | BMI: 15–25% (12 mo) |
Picrosirius red (Col I+III) Fibrosis score ≥ 2 VCTE stiffness scAT-liver correlation (ρ = 0.14) |
No fibrosis ↓ post-BS ↑ Baseline fibrosis → ↓ WL Fibrosis predicts outcome Associated: age, T2D, ↑BMI/IL-6 |
| McCulloch et al. 2015 [78] |
Human obese BS (n = 16) |
scAT | 12 mo post-BS-op | 20–30% |
COL6A3, COL1A1 ECM gene |
COL6A3: leptin-regulated COL6A3 ↑ after WL ↓ AT fibrosis |
| Divoux et al. 2010 [69] |
Human obese BS (n = 65) Lean controls (n = 9) |
scAT Omental AT |
0, 3, 6, 12 mo post-BS-op | 30–35% |
Col I, III, VI Pericellular/total fibrosis |
↑ Baseline fibrosis → ↓ fat mass loss Modest fibrosis ↓ Persistent in some pts Limits metabolic improvement |
| Liu et al. 2016 [79] |
Human obese BS (n = 118) |
scAT | 12 mo post-op | ~30% | COL I/III/VI, LOX, MMPs, degraded collagens |
↑ Collagen degradation, ↓ cross-linking → adequate ECM remodeling |
| Osorio-Conles et al. 2023 [76] |
Human obese BS (n = 144) |
scAT, VAT | 12 mo post-BS-op | ~30% | COL1A1, COL5A1, COL6A3 | scAT ECM gene expression predicts WL; ↑COL5A1/COL6A3 → ↓WL |
| Chabot et al. 2017 [72] |
Human obese BS (n = 35, including n = 12 T2D) |
scAT, VAT | 6 mo post-BS-op | ~20–25% | Masson’s trichrome, COL1A1/3A1/6A3, LOX | High baseline fibrosis in T2D; no resolution at 6 mo; fibrosis persists despite WL |
| Ledoux et al. 2022 [74] |
Human obese BS (n = 161) |
scAT, VAT | Baseline, 1 yr, 3 yr post-BS-op | ~30% |
Stromal adipogenic/ myofibrogenic progenitors CD4+ T cells Col/fibrosis markers |
↑ scAT progenitors + VAT hypertrophy → ↓ WL Persistent fibrotic phenotypes → less favorable outcomes |
| García-Rubio et al. 2018 [73] |
Human obese BS (n = 43 pre-BS, n = 28 post-BS) |
scAT VAT (flow cytometry) |
Baseline, 1 yr post-BS-op | 30–50% |
CD34+ APC Col staining ECM markers |
↑ Progenitor cells ↑ Regenerative capacity Modest fibrosis ↓ Persistent ECM barriers |
| Camastra et al. 2017 [75] | Human obese (n = 13 T2D, n = 15 ND) + 9 lean controls | scAT, VAT RAM | Baseline, 12 mo post-BS-op | ~33% |
Histological fibrosis/necrosis, CLS Irregular capillary BM Col staining |
Partial persistence of fibrotic changes Relatively irreversible VAT fibrosis → β-cell dysfunction ↓ Systemic metabolis |
AT, adipose tissue; APC, adipocyte progenitor cells; BM, basement membrane; BMI, body mass index; BS, bariatric surgery; BW, body weight; CLS, crown-like structures; Col, collagen; d, day; ECM, extracellular matrix; IL-6, interleukin-6; LOX, lysyl oxidase; MMP, matrix metalloproteinase; mo, month; post-op, post-operative; pts, patients; RAM, rectus abdominis muscle; scAT, subcutaneous adipose tissue; SPARC, secreted protein acidic and rich in cysteine; T2D, type 2 diabetes; ND, non-diabetes; VAT, visceral adipose tissue; VCTE, vibration-controlled transient elastography; VLCD, very-low-calorie diet; WG, weight gain; WL, weight loss; wk, week; yr, year; ↑, increase/ increased/higher; ↓, decrease/decreased/lower; ~, approximately.
Table 3
| Study | Model | Sample type | Treatment duration | Weight loss % | Fibrotic markers | Key findings |
|---|---|---|---|---|---|---|
| Michailidou et al. 2012 [81] | Mouse (HSD1- deficient & wildtype) | Epididymal adipose tissue | 20 weeks HFD | NC | HIF-1α, TGF-β, Smad3, α-SMA, collagen, MMP14 | ↑ Angiogenesis & microvascular density in HSD1-deficient mice → ↓ hypoxia → ↓ fibrotic markers → protection against fibrosis |
| Watanabe et al. 2016 [82] | Mouse (HFD-induced obesity) | Epididymal adipose tissue | 20 weeks HFD ± Isoliquiritigenin | NC | Collagen (histology), TGF-β, α-SMA, Mincle, COL1, COL3, MMPs | Isoliquiritigenin: ↓ HFD-induced fibrosis & inflammation via inhibiting innate immunity (NF-κB, TLR4, Mincle, NLRP3) |
| Kohda et al. 2025 [28] | Mouse (HFD-induced obesity) | Epididymal adipose tissue (scRNA-seq & spatial transcriptomics) | 16–20 weeks HFD | NS | Mincle, OSM, COL1A1, TGF-β, PDGF, ACTA2 (α-SMA), ECM/ collagen | Macrophage-fibroblast via Mincle-OSM axis → fibrosis regulation; OSM deficiency → ↑ fibrosis (multi-cell process) |
| Saha et al. 2025 [50] | Mouse (HFD-induced obesity) | Subcutaneous & inguinal WAT | 20 weeks HFD | NS | PAI-1, COL1A1, COL5A3, TGF-βR3, SAA3, Sirius red, hydroxyproline, ECM markers | miR-30a ↓ fibrosis via PAI-1 repression → maintained insulin sensitivity → healthy WAT expansion without metabolic dysfunction |
ACTA2, actin alpha 2, smooth muscle; α-SMA, alpha-smooth muscle actin; COL, collagen; ECM, extracellular matrix; HFD, high-fat diet; HIF-1α, hypoxia-inducible factor 1-alpha; HSD1, hydroxysteroid dehydrogenase type 1; miR, microRNA; MMP, matrix metalloproteinase; NC, no change; NF-κB, nuclear factor kappa B; NLRP3, NOD-like receptor family pyrin domain containing 3; NS, not significant; OSM, oncostatin M; PAI-1, plasminogen activator inhibitor-1; PDGF, platelet-derived growth factor; SAA3, serum amyloid A3; scRNA-seq, single-cell RNA sequencing; Smad3, mothers against decapentaplegic homolog 3; TGF-β, transforming growth factor-beta; TGF-βR3, transforming growth factor-beta receptor 3; TLR4, toll-like receptor 4; WAT, white adipose tissue;↓, decreased; ↑, increased.
Table 4
| Study | Model | Sample type | Treatment duration | Weight loss % | Fibrotic markers | Key findings |
|---|---|---|---|---|---|---|
| Sato et al. 2018 [87] | Human (T2DM + CAD, n = 40) | EAT | 6 months, dapagliflozin 10 mg/ day | ~4.0% | ↓EAT volume, ↓plasma TNF-α, ↓PAI-1 | ↓EAT volume, ↓inflammation (TNF-α, PAI-1), improved metabolic parameters |
| Sato et al. 2020 [88] | Human (T2DM + CAD, n = 35) | EAT | 6 months, dapagliflozin 10 mg/ day | ~4.2% | ↓EAT volume, ↓TNF-α, adipo-fibrokines (TGF-β) | ↓EAT volume, ↓TNF-α, improved P-wave dispersion, modulated atrial fibrosis mediators |
| Checa-Ros et al. 2025 [90] | Mouse (HFD) + Human EAT explants | AT (mouse), EAT (human) |
Mouse: 12–20 weeks HFD + empagliflozin Human: ex vivo dapagliflozin 24–72 h |
Significant ↓body weight, ↓AT mass | Mouse: ↓TNF-α, ↓IL-6, ↓MCP-1, ↓NLRP3 (IL-1β, IL-18), ↓TGF-β | ↑Lipolytic enzymes, ↓visceral adiposity (mice), ↓pro-fibrotic factors (human EAT) |
| Takano et al. 2023 [89] | Human (n = 25, non-diabetic) | Epicardial preadipocytes | 19 days in vitro, empagliflozin 0–100 μmol/L | N/A (ex vivo) | ↓IL-1α, ↓IL-1β, ↓IL-6, ↓TGF-β1, ↓MCP-1, ↓FABP4 | ↓Adipogenesis, ↓pro-fibrotic adipokine secretion, ↓inflammatory/fibrotic genes |
AT, adipose tissue; CAD, coronary artery disease; EAT, epicardial adipose tissue; FABP4, fatty acid binding protein 4; HFD, high-fat diet; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; N/A, not applicable; NLRP3, NOD-like receptor family pyrin domain containing 3; PAI-1, plasminogen activator inhibitor-1; SGLT2, sodium-glucose cotransporter 2; T2DM, type 2 diabetes mellitus; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; ↓, decreased; ↑, increased; ~, approximately.
Table 5
| Study | Drug | Model | Sample type | Treatment duration | Fibrotic markers | Key findings |
|---|---|---|---|---|---|---|
| Spencer et al. 2014 [59] | Pioglitazone | Human (IR, IGT, n = 9) | scAT | 12 weeks, 45 mg/day |
Total COL: No change Elastin: 6-fold ↑ (p < 0.05) ECM via Elastic Stain |
↓ Total macrophages 26% ↓ M1 macrophages 56% ↑ Elastin fibers Improved ECM flexibility |
| Matsuura et al. 2015 [95] | Pioglitazone | Rat (DS/obese Mets) | VAT & scAT, Cardiac tissue | 4 weeks, 2.5 mg/kg/day (9–13 weeks age) |
LV fibrosis markers Adipocyte hypertrophy TNF-α, MCP-1 Adiponectin AMPK phosphorylation |
↑ Obesity but ↓ LV hypertrophy ↓ Fibrosis ↓ Diastolic dysfunction |
| Khan et al. 2009 [94] |
PPARγ agonist COOH |
col6KO mice col6KO ob/ob mice Wild-type mice |
AT (epididymal, mesenteric) | 14 days, 10–30 mg/kg (10-week-old) |
COL1, COL3, COL6 TGF-β1 α-SMA SMAD2/3 |
↓ COL expression ↓ TGF-β1/SMAD2/3 signaling Weakened ECM scaffold Improved metabolic phenotype |
| Silveira et al. 2025 [96] | Pioglitazone | Mouse (C57BL/6, doxorubicin-treated) | White AT (iWAT, vWAT) | Co-administration with doxorubicin |
Lipolysis markers Adipokines F4/80, CD11c Metabolic parameters |
Protected inguinal adipose from doxorubicin damage ↓ Inflammatory responses ↓ Immune cell infiltration |
AMPK, AMP-activated protein kinase; α-SMA, alpha-smooth muscle actin; AT, adipose tissue; COL, collagen; col6KO, collagen VI knockout; COOH, carboxyl group; DS, Dahl salt-sensitive; ECM, extracellular matrix; IGT, impaired glucose tolerance; IR, insulin resistance; iWAT, inguinal white adipose tissue; LV, left ventricular; M1, classically activated macrophage; MCP-1, monocyte chemoattractant protein-1; Mets, metabolic syndrome; ob/ob, obese mice with leptin gene mutation; PPARγ, peroxisome proliferator-activated receptor gamma; scAT, subcutaneous adipose tissue; SMAD, mothers against decapentaplegic homolog; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; VAT, visceral adipose tissue; vWAT, visceral white adipose tissue; ↑, increase/increased/higher; ↓, decrease/decreased/lower.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



