INTRODUCTION
Membranous nephropathy (MN) is a common cause of nephrotic syndrome in adults [
1] characterized by the deposition of immune complexes in subepithelial space that result in the thickening of the glomerular basement membrane [
2]. Idiopathic MN (iMN) accounts for 80% of all MN cases [
3]. Secondary MN (sMN), mainly caused by infection, neoplasm, autoimmune disease, and drugs, causes the other 20% [
3].
iMN is associated with autoantibodies mainly against the podocyte antigen M-type phospholipase A2 receptor (PLA2R) [
4]. In approximately 70% to 80% of patients with iMN, anti-PLA2R antibody (anti-PLA2R-Ab) is found in serum [
4]. PLA2R in kidney tissue is also detected in patients with iMN. Therefore, the presence of serum anti-PLA2R-Ab and histological PLA2R in kidney tissue strongly suggests iMN [
5,
6].
Regarding prognosis, the achievement of remission is highly important for improving renal and patient survival [
7]. Similarly, relapse or persistence of proteinuria is a negative prognostic factor on renal survival [
8].
The treatment of MN begins with supportive care with use of diuretics, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ARBs), and lipid-lowering agents aimed to control edema, blood pressure, or other complications of nephrotic syndrome. However, even with the best supportive therapy, full-blown nephrotic syndrome is difficult to control, especially in patients with multiple risk factors for progression, which influences prognosis; these factors include the degree of proteinuria and kidney function, lower molecular weight proteinuria, and the levels of anti-PLA2R-Ab. Immunosuppressive (IS) therapy should only be started in subgroups with a higher risk for progression to determine the optimal balance between benefit and harm, especially due to the side effects of conventional IS agents including steroids, cyclosporine A, tacrolimus, and mycophenolate mofetil, cyclophosphamide.
Understanding of the pathogenesis of iMN leads to the development of B-cell targeting therapy [
9,
10]. Rituximab (RTX), a monoclonal antibody against the CD20 antigen present in B lymphocytes, has been used as alternative to the abovementioned IS agents [
11]. Since the first clinical study by Remuzzi et al. [
12], it has been raised as a promising therapy for iMN. Based on previous studies, the average remission rate of proteinuria after RTX treatment is more than 65% to 90% using different treatment schedules [
13]. However, the outcomes were mainly derived from Caucasian patients and it seems to be affected by ethnicity or genetic background as well as a different treatment protocol. From a Chinese study by Wang et al. [
14], RTX has induced complete or partial remission in 41.7% of nonresponsive patients with iMN, but it was limited by a small sample size and nonunified protocol. A treatment protocol modified for Asian or Korean patients remains to be established.
Here, we describe our experience in treating patients with RTX in iMN who had severe proteinuria and failed to respond to prior IS therapies from a single-center university-based tertiary hospital in Korea with a literature review.
METHODS
Patients
Fifteen patients with iMN and severe proteinuria were treated with RTX in the Kyungpook National University Hospital from January 2014 to July 2020. We excluded one patient with estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 at initial treatment and one patient treated with RTX as a first IS agent. We included patients (n = 13) who met the following criteria: biopsy-proven iMN; age of at least 18 years; severe proteinuria (Ōēź 4 g/day); and failure to respond to prior IS agents including cyclosporine A, tacrolimus, mycophenolate mofetil, and steroid. All patients received RTX as a rescue therapy. Appropriate serological investigations were conducted for all patients with MN to exclude viral or bacterial infection, autoimmune diseases, and malignancy. Anti-nuclear antibody, anti-dsDNA antibody, complements, hepatitis B surface antige and, anti-hepatitis C virus antibody, venereal disease research laboratory test, human immunodeficiency virus Ag/Ab, and C-reactive protein (CRP) were negative in all patients. Drug-related sMN was excluded by reviewing the medical history of the patient. Gastrointestinal endoscopy and radiological investigations, such as abdominal computed tomography or ultrasound, were performed on our patients to rule out malignancy.
This study was approved by the Institutional Review Board of Kyungpook National University Hospital (IRB No. 2020ŌĆō07ŌĆō034). Written informed consent by the patients was waived due to a retrospective nature of this study.
Treatment protocol and follow-up
Treatment of RTX is not covered by the Korean Health Insurance System, and it was used only as off-labeled after approval from the Korean Food and Drug Administration. Therefore, we have established this treatment protocol based on both the efficacy from previous studies with different protocols and the cost. This administration protocol mainly consists of one cycle of two RTX intravenous infusions of 375 mg/m2 at a 2-week interval. However, due to the retrospective nature of this study, the exact doses were determined by the judgment of each physician in charge of the patients.
RTX infusion was always preceded by the administration of methylprednisolone (100 mg intravenously in 100 mL sodium chloride 0.9% over 1 hour, 3 hours prior to RTX), paracetamol (650 mg orally 30 minutes prior to RTX) and fexofenadine (180 mg orally 30 minutes prior to RTX) to prevent possible allergic reactions. RTX was constituted in 5% dextrose 500 mL and was infused at an initial rate of 50 mL/hr, then increased to 100 mL/hr if tolerated. The continued use of other IS agents in each patient depended on the clinical decision of each physician.
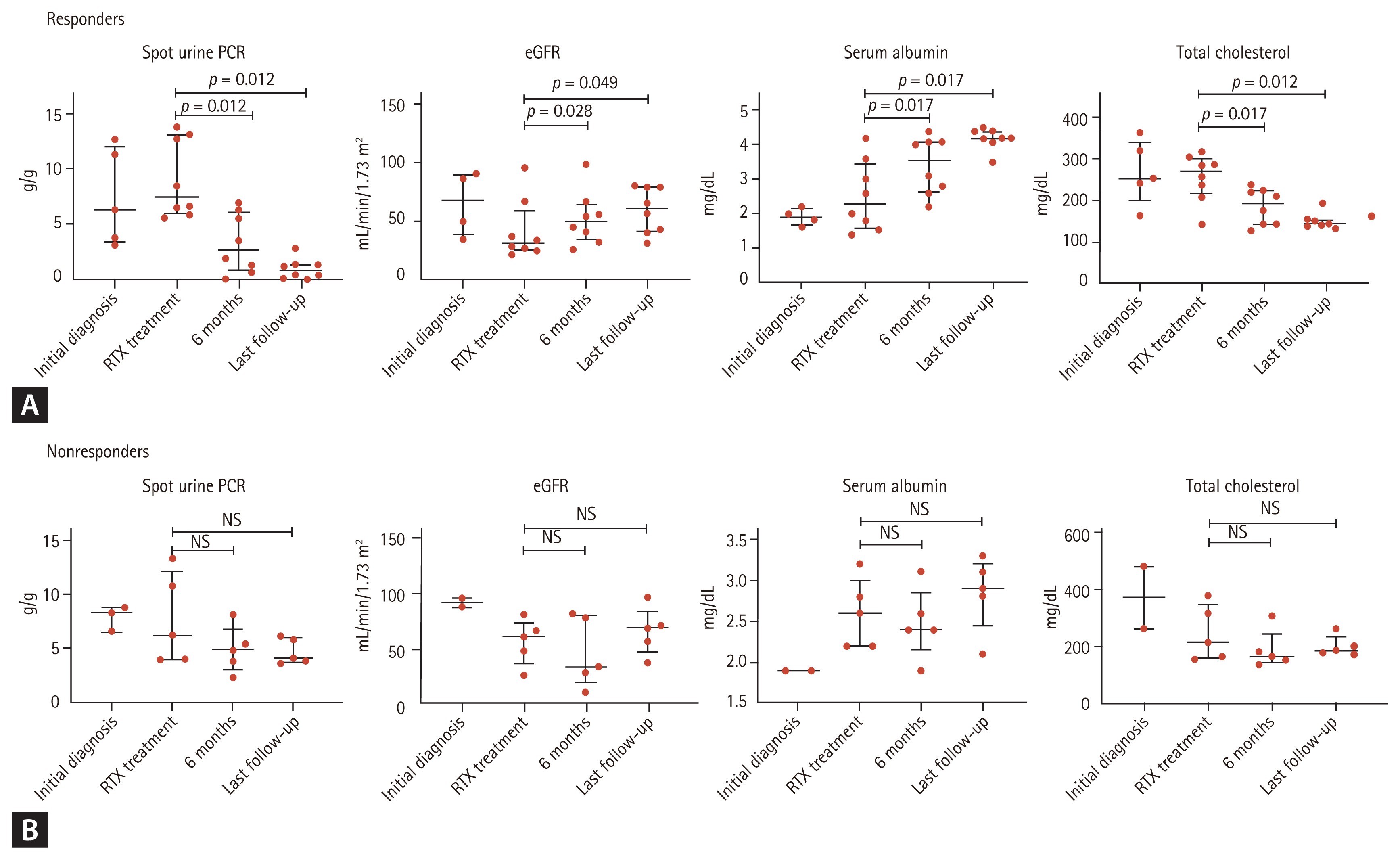
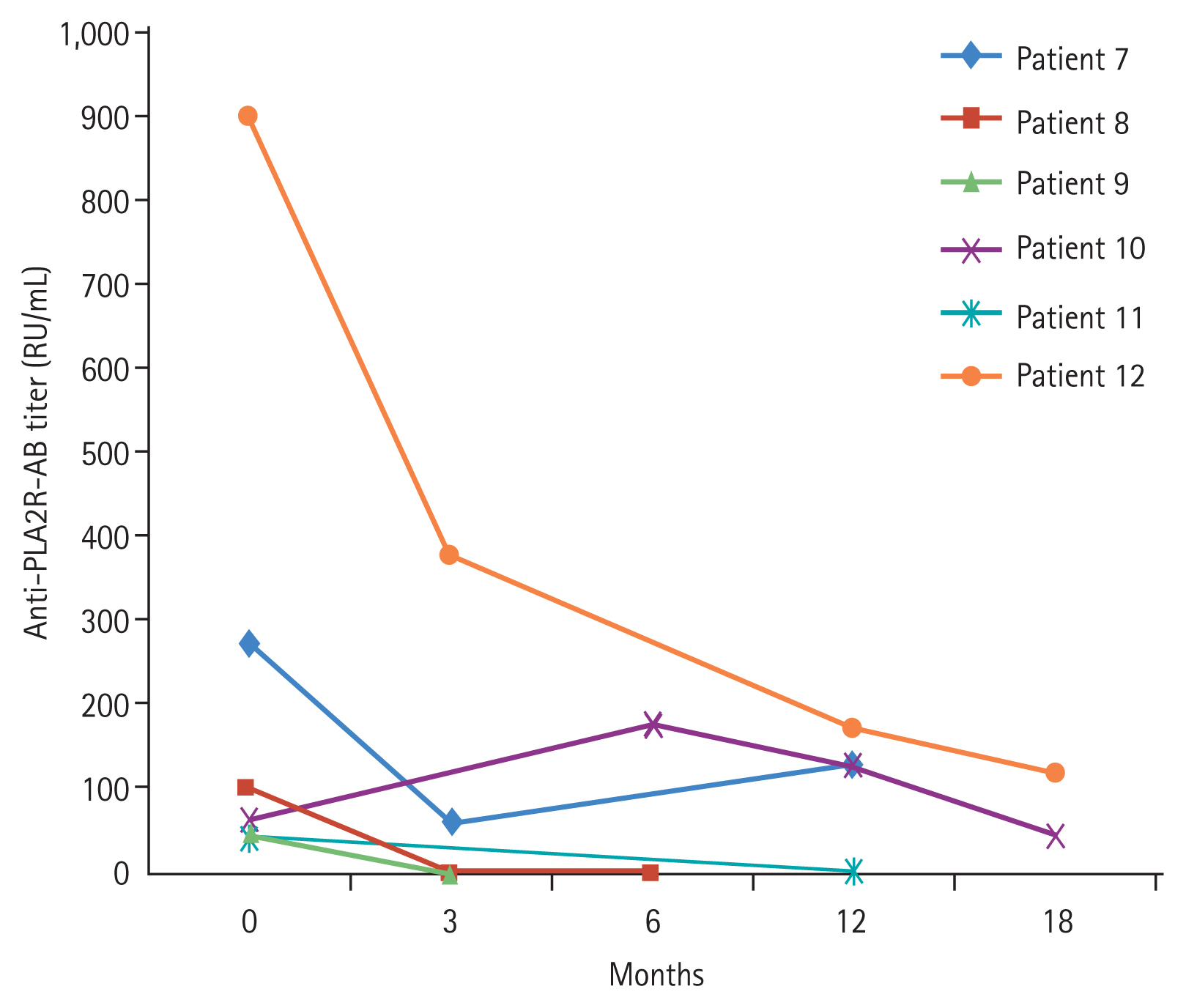
The response rate was evaluated up to 71 months (median, 22 months; interquartile range [IQR], 17.0 to 56.0). Clinical and biological data at diagnosis, at RTX initiation, at every 3 months after RTX, and at last follow-up were retrospectively recorded. In each follow-up, we measured proteinuria as spot urine protein-to-creatinine ratio (PCR), serum creatinine, urea, and albumin. We measured the circulating anti-PLA2R-Abs and the cluster of differentiation 19 (CD19) expressed on the surface of B lymphocytes. For the detection of anti-PLA2R-Abs, enzyme-linked immunosorbent assay (Seoul, Korea) was used. Antibody positivity was indicated by > 14 RU/mL.
Outcome measures
The main outcome measures were the percentage of patients who achieved complete remission or partial remission at 6 months, 12 months, and last follow-up (median, 22 months; IQR, 17.0 to 56.0).
Based on Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines, partial remission in this study was defined as urine PCR < 3.5 g/g with at least a 50% reduction from baseline and stable renal function, and complete remission as urine PCR < 0.3 g/g [
15]. Patients who did not meet these values were considered non-responders. Relapse was defined as a recurrence of proteinuria >3.5 g/day after a period of partial or complete remission. Immunologic remission was defined as negative for circulating anti-PLA2R-Ab detection.
Statistical analysis
Data were presented as mean, median (IQR), and percentage as appropriate. For comparison of initial characteristics between responder and non-responder groups, we used a nonparametric MannŌĆōWhitney U test. We analyzed changes of several outcomes before and after RTX in each group using the Wilcoxon tests. Statistical analysis was performed using IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA). For this analysis, a p < 0.05 was considered statistically significant.
DISCUSSION
In this study, we investigated the efficacy and safety of RTX treatment in patients with iMN who were refractory or relapsed after treatment with other IS agents in a case series. RTX treatment achieved complete or partial remission in 38.5% of patients in 6 months and 61.5% in the last follow-up with no significant safety issues.
Treatment outcomes, standard doses, and protocol of RTX treatment varied between countries and hospital units. In addition, relevant data of RTX for Asian patients have been scarce, despite the recent KDIGO recommendation for RTX in patients with iMN [
15]. Therefore, we comparatively reviewed the results of RTX treatment from previous studies, including studies from Asia in
Table 4.
Ruggenenti et al. [
16] showed clinical outcomes of RTX treatment in 100 patients with iMN. Of them, 68 patients were treated with RTX as first-line therapy and 32 patients with RTX as second-line therapy for other immunosuppressants. In all patients, the median creatinine and median proteinuria before RTX were 1.2 mg/dL and 9.1 g/day, respectively. RTX (375 mg/m
2) was administered weekly for 4 consecutive weeks. After the first infusion of RTX, patients with over five circulating B-cells per mm
3 received a second infusion. In a median follow-up duration of 29 months, 65 patients (65%) achieved complete or partial remission. Treatment-related serious adverse effects were not observed.
Fiorentino et al. [
17] included 38 patients with iMN treated with RTX, of which 13 received RTX as first-line therapy, and the remaining 25 who received RTX as second-line therapy. RTX (375 mg/m
2) was administered by intravenous infusion weekly for 4 consecutive weeks. In both groups, baseline median serum creatinine was 1.1 mg/dL. Median 24-hour proteinuria was 5.4 g in the first-line group and 6.3 g in the second-line group. Complete remission was achieved by 15 patients (39.5%), and partial remission was achieved by 14 patients (36.8%) during a median follow-up duration of 15 months. Serious adverse event data were not reported.
Besides earlier observational studies, more recent randomized controlled trials with RTX have shown positive results of RTX. Dahan et al. [
18] randomly allocated patients to nonimmunosuppressive antiproteinuric treatment (NIAT) plus RTX administration (375 mg/m
2) at day 1 and 8 or NIAT alone after 6 months of non-IS treatment. In NIAT plus RTX, median serum creatinine and median urine PCR at baseline were 1.1 mg/dL and 7.7 g/g, respectively. In the only NIAT group, median serum creatinine and median urine PCR were 1.0 mg/dL and 7.2 g/g, respectively. Remission rates were higher in NIAT plus RTX (64.9%) group than only NIAT group (34.2%) (
p < 0.01). The rates of anti-PLA2R-Ab depletion were higher in the NIAT plus RTX than NIAT groups at 3 months (56% vs. 4.3%) and 6 months (50% vs. 12%). In each group, eight serious adverse events were developed.
Wang et al. [
14] has shown the efficacy and safety of RTX in 36 Chinese patients who were nonresponsive to prior immunosuppressants. According to the physicianŌĆÖs discretion, 36 patients were managed with RTX. Baseline mean creatinine and proteinuria in 36 patients were 2.1 mg/dL and 12.3 g/day, respectively. During follow-up (median, 12 months), 15 of 36 patients (41.7%) achieved partial (n = 13, 36.1%) or complete (n = 2, 5.6%) response to RTX. Only one patient experienced severe adverse events.
With a limited use of IS agents in patients with decreased renal function with eGFR < 30 mL/min/1.73 m
2, Hanset et al. [
19] described the outcome of RTX treatment in an advanced stage of chronic kidney disease (CKD) in 13 patients with PLA2R-associated MN at stage 4 or 5 CKD treated with RTX. The regimen consisted of either 2-weekly doses of 375 mg/m
2 or two RTX infusions of 1 g/day at a 2-week interval. The treatment protocol was repeated as needed to induce PLA2R-Ab complete depletion. The mean eGFR and urinary protein at RTX initiation were 18 mL/min/1.73 m
2 and 13.2 g/day, respectively. Over a median follow-up period of 17.8 months, nine of 13 patients achieved complete or partial remission. Immunological remission occurred in 10 patients, but one of them required chronic hemodialysis. Three patients experienced severe adverse events.
Yoon et al. [
20] reported the experience of using RTX in two previously treated patients with combinations of IS agents. Because previous immunosuppressant agents were nonresponsive, both patients received two cycles of RTX for the following treatment. One cycle of RTX consisted of 1 g infusion on days 1 and 15. In both cases, their proteinuria decreased and partial remission was achieved without side effects.
Compared with previous studies, in this study, the total remission rate (61.5%) was relatively lower, except that of Wang et al. [
14]. It is probably because all patients in our cases had severe proteinuria greater than equal to 4 g/day and lower eGFR compared with other previous studies. Furthermore, the condition of all patients worsened despite previous IS therapy prior to initiation of RTX. A high level of proteinuria at RTX therapy and resistance to other immunosuppressants indicate poor renal outcome and high disease activity of MN. Some patients had a score of at least 2 in the tubular atrophy or interstitial fibrosis core. A higher score is associated with worse baseline eGFR and poor renal outcome. In addition, the higher score of tubular atrophy or interstitial fibrosis score, the more likely it is to not respond to RTX.
A lower dose of RTX than that used in other studies may partly be the reason for a lower response rate. Few data on RTX dosing protocol for iMN in Asian patients are available. We adopted a lower dose of RTX based on a previous study in KT, which showed similar effectiveness for a lower dose of RTX in ABO-incompatible KT [
21]. Moreover, the cost of RTX treatment is not covered by the National Health Insurance system for MN treatment in Korea. Remission rate of 61.5% is not too low considering that all patients had high risk of progression. Further studies are necessary to provide a strategy for more cost-effective RTX therapy than the standard regimen, especially in the Asian population.
Although side effects have been reported in other studies, a generally good tolerability of RTX is demonstrated in our study with no side effects related to RTX in all patients. This may also be related to the lower dose and 2-week interval of our protocol. RTX is more expensive compared with other therapies for iMN in Korea because of the lack of insurance coverage. If we treat a patient with MN weighing 60 kg for 6 months, we assume that the dose of each IS administration for 6 months is set as in KDIGO guidelines. Treatment with cyclophosphamide is the least expensive. The cost of mycophenolate mofetil treatment is approximately 12-fold higher than cyclophosphamide, calcineurin inhibitors (CNIs) are approximately 23 to 25-fold higher, and RTX is 37-fold higher than cyclophosphamide. RTX, though expensive on its own, may be potentially more cost-effective and safe than other IS agents [
22]. Other IS drugs, especially cyclophosphamide, increase the risk of infection, malignancy, and bone marrow suppression, and CNIs have a risk of renal dysfunction. In view of side effects associated with other immunosuppressants, RTX is superior to other IS drugs. When using RTX, high acquisition cost might be offset by lower adverse events and higher response rates than using other immunosuppressants. RTX also reduced oral pill-burden compared to other IS therapies.
Anti-PLA2R-Ab is a specific marker for iMN [
23,
24]. Anti-PLA2R-Ab titers have a significant correlation with iMN activity and could also be useful in the prediction of disease outcome [
25]. Serial monitoring of anti-PLA2R-Ab appears to be helpful in evaluating the response for RTX and determining the timing of retreatment with RTX. RTX therapy leads to a decline in serum anti-PLA2R-Ab [
26]. In other words, RTX can help achieve complete immunological remission. It suggests that patients with a higher titer of anti-PLA2R-Ab might benefit from higher doses of RTX. It is necessary to modify the RTX doses according to the anti-PLA2R-Ab titer. However, not all patients with MN are positive for anti-PLA2R-Ab; therefore, efforts to find other antibodies such as anti-thrombospondin type 1 domain-containing 7A (THSD7A) should be continued.
This study has several limitations. This is a retrospective study with a small number of patients. The total RTX dose derived from body surface area was not equal in all patients. In addition, this study was short to capture long-term side effects of RTX. Furthermore, we are unable to collect sufficient baseline data such as histologic characteristic, CD19, and anti-PLA2R-Ab. Another limitation of this study is the lack of information on changes in peripheral blood CD19/20+ B-cell counts following treatment with RTX.
After the discovery of PLA2R as the target antigen in iMN, THSD7A, neural epithelial growth factor-like 1-protein, protocadherin-7, and exotosin1/exotosin2 were also characterized as causative antigens for MN. We could not measure the antibodies to these antigens due to the limitation of commercialized detection techniques for these antigens.
In conclusion, in Korean patients with iMN who are refractory to other IS agents, RTX induces clinical remission in 61.5% of high-risk iMN without adverse effects. Individualized therapy plan including dose or timing of RTX based on anti-PLA2R-Ab and B-cell counts might be needed to prolong the remission status or reduce relapse in iMN.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



