DISCUSSION
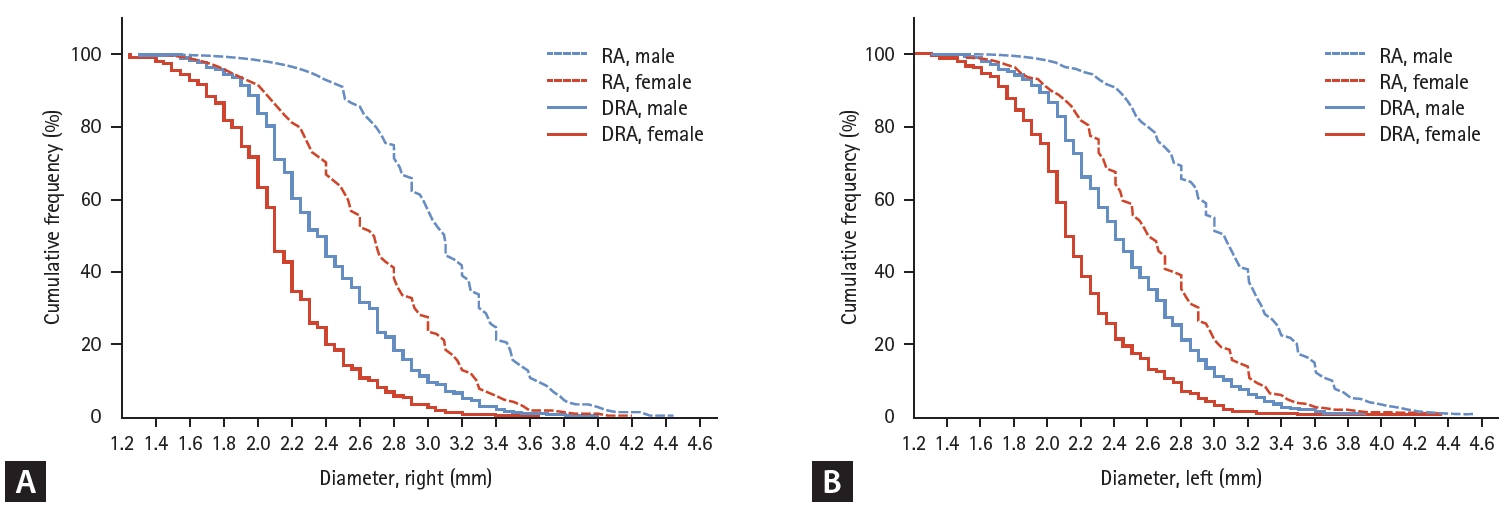
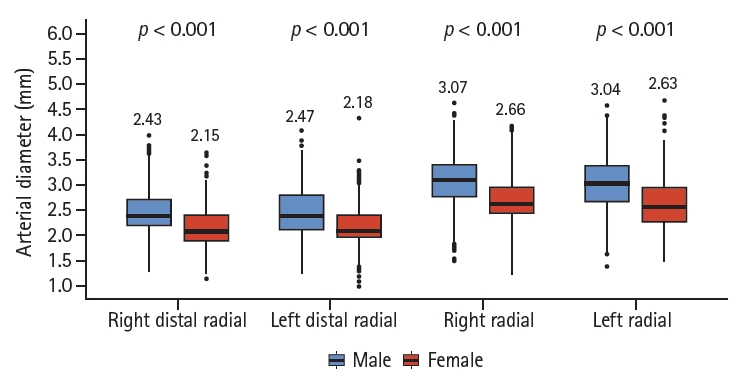
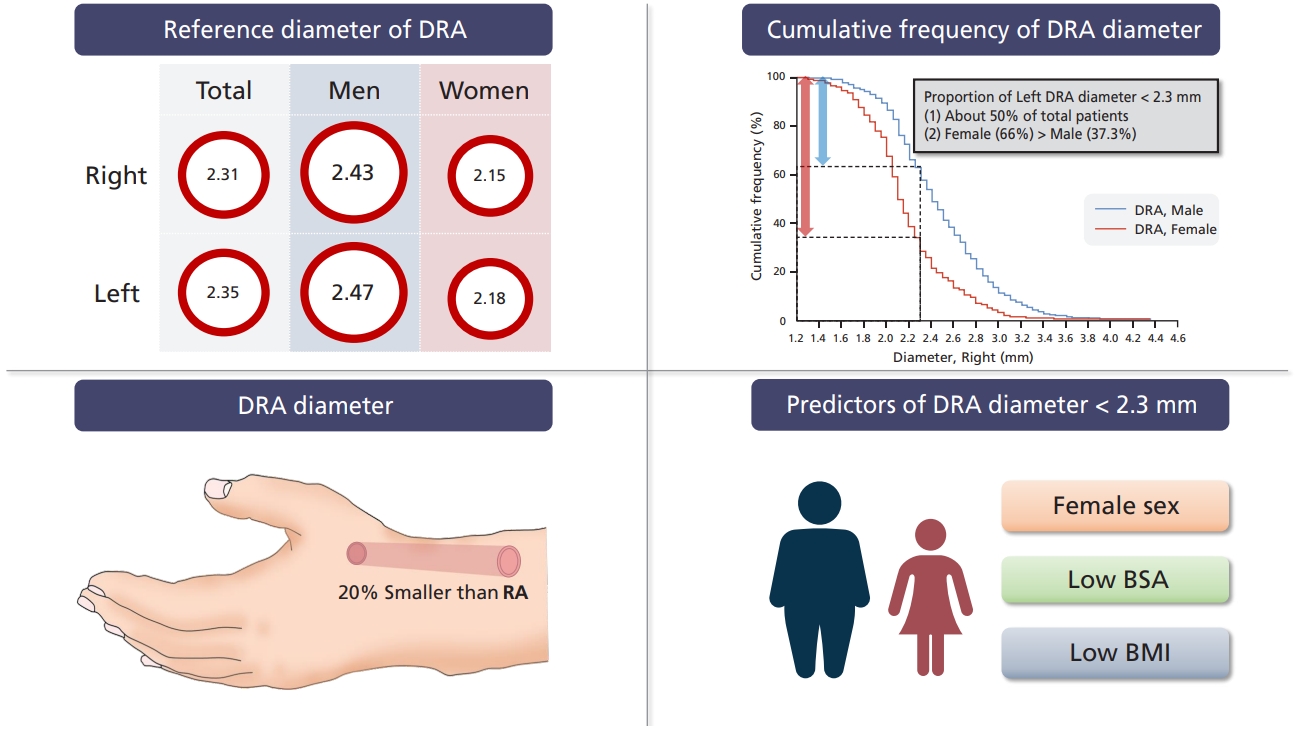
The main aim of this study was to determine the DRA reference diameter in Korean patients. The mean DRA diameters were 2.31 ± 0.43 mm (right) and 2.35 ± 0.45 mm (left). Women had a smaller DRA than men (2.15 ± 0.38 mm vs. 2.43 ± 0.44 mm in the right hand; 2.18 ± 0.39 mm vs. 2.47 ± 0.45 mm in the left hand). The other major findings were as follows: (1) DRA diameter was approximately 20% smaller than RA diameter; (2) female patients had a smaller DRA diameter than male patients; (3) approximately 50% of patients had a small DRA (< 2.3 mm); and (4) female sex, low BMI, and low BSA were predictors of a DRA diameter < 2.3 mm.
Several studies have reported on DRA diameter (
Table 5). Mizuguchi et al. [
8] evaluated the DRA at two available puncture points (within and outside the anatomical snuffbox [on the dorsum of the hand]) using ultrasonography in 228 patients. The measured DRA diameter was similar to that observed in the present study. The DRA increased in size the day after the procedure and then returned to the baseline measurement after 1 month. The DRA diameter in the anatomical snuffbox was greater in a Japanese study than that observed in our study [
9]. Based on the quantitative coronary angiography data obtained by Kim et al. [
6], the DRA size after the injection of spasmolytics (nitroglycerin and verapamil) was similar to that reported by Norimatsu et al. [
9] and was greater than that noted in our study. The smallest DRA diameter was reported by Naito et al. [
10] (2.04 ± 0.60 mm in men and 1.96 ± 0.44 mm in women). The angiographic evaluation of 52 patients from Italy revealed a mean DRA diameter of 2.22 ± 0.14 mm after the administration of a vasodilatory cocktail containing 2.5 mg of verapamil, 1 mL of lidocaine hydrochloride, and 1 mL of bicarbonate [
11]. The inconsistent values may be attributed to disparate definitions of vessel diameter and different points of measurement among investigators. Naito et al. [
10] measured the vessel diameter of the tunica media, while we measured the outer surface of the vessels because the ultrasonographic resolution was not optimal to clearly define the inner wall of the DRA. The anatomical landmarks for the measurement of vessel diameter may also differ among investigators. To the best of our knowledge, our study included the largest number of patients to evaluate the DRA diameter. Furthermore, this study provides information on the DRA and RA diameters.
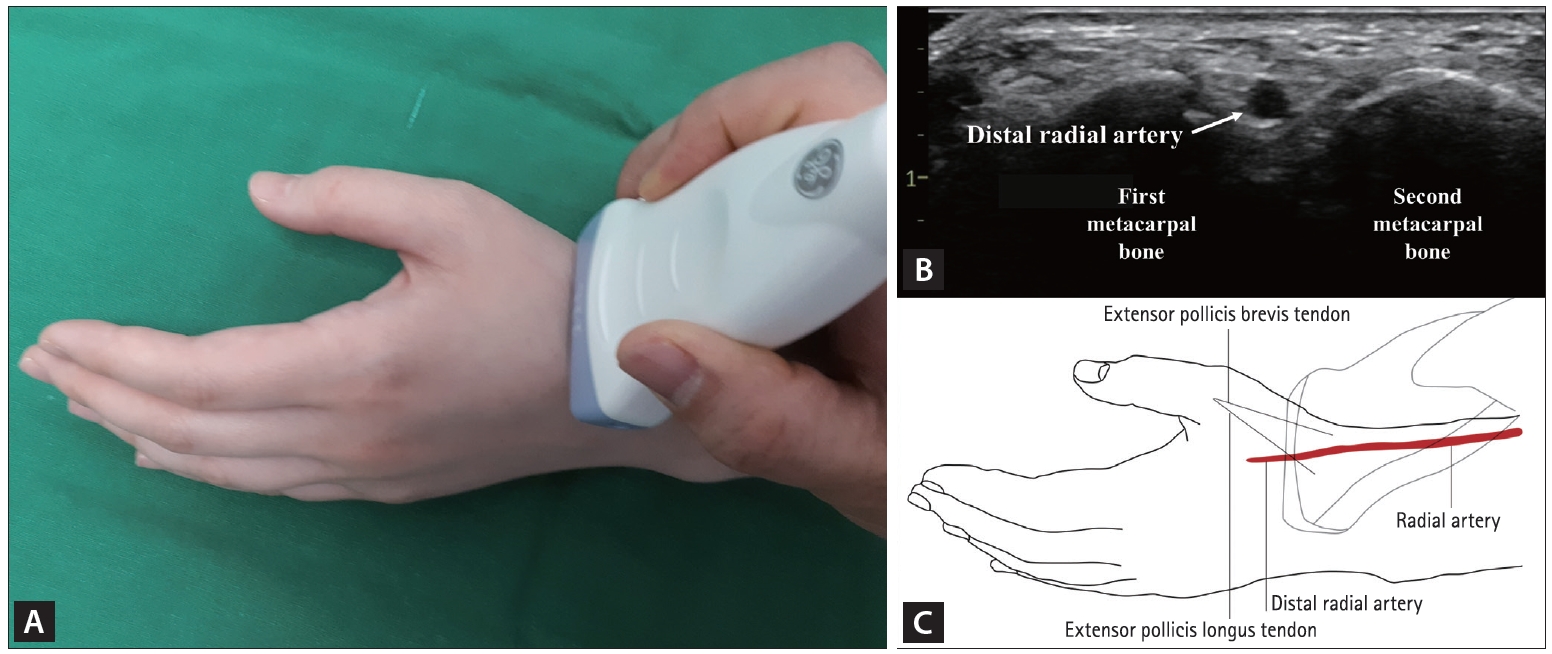
There is no consensus regarding the ideal method for assessing DRA diameter. The arterial diameter may be affected by emotional status, room temperature, vasospasm during skin preparation with cold antiseptic agents (povidone-iodine, betadine, and chlorhexidine gluconate), the use of spasmolytics before measurement, ultrasonographer’s technical skills, and discordance between ultrasonography and angiography measurement methods. Accordingly, a systematic protocol for assessing DRA diameter from the time of adopting the initial approach during follow-up, especially using ultrasonography, would aid in popularizing this novel approach based on the anatomic and physiological rationale [
12,
13]. Of note, the diameter of the actual punctured site of the DRA may be equal to or greater than the measured value because the needle usually punctures the vessel wall in an area proximal to the measured area [
8].
In this study, the DRA diameter was apparently associated with a low BMI and a low BSA. BMI was significantly correlated with DRA diameter (
r = 0.66,
p < 0.0001) in a Japanese study of 142 patients [
9]. However, another Japanese study of 120 patients did not report an association between vessel diameter and clinical variables, including BMI, in multivariate analysis [
10]. In our study, a low BMI was a significant predictor of a DRA diameter < 2.3 mm in both hands. Moreover, female sex and a low BSA were strong predictors of a DRA diameter < 2.3 mm. The association between sex and RA diameter was reported in several studies. In a large-scale study evaluating RA diameter that included 1706 patients from Indonesia, India, and Macedonia, female sex was associated with a small RA (diameter < 2.8 mm) (OR, 1.72; 95% CI, 1.40 to 2.12;
p < 0.001); however, a BMI < 25 kg/m
2 was not a predictor for a small RA (OR, 1.0; 95% CI, 0.82 to 1.22;
p = 0.93) [
14]. A study conducted of a population in the United States (n = 175) reported that female sex was the only predictor of a small RA diameter [
15]. Data showing an association between a low BSA with a small DRA are not available except in the current study. However, there were no sex-based differences in the indexed DRA diameter by BSA. It is worth remembering the guideline for echocardiography, which states that the cardiac volume, dimension, and valve area should be indexed with BSA for comparison with individuals with different body sizes [
16-
18]. From this viewpoint, a smaller DRA may be present in women with a small body size, indicating a low BSA.
A small DRA diameter is a natural limitation that should be overcome for routine CAG and PCI. An international consensus paper on preventing radial artery occlusion (RAO) after transradial angiography and interventions reported that RAO was significantly associated with an RA/sheath ratio < 1 as well as female sex, a low BMI, age, and ethnicity [
19]. Miniaturized devices with a hydrophilic coating, such as the sheathless guiding catheter (Asahi Intecc, Nagoya, Japan) and the Glidesheath slender sheath (Terumo, Tokyo, Japan), may increase the possibility of using the distal radial access in patients with a small DRA and reduce the risk of DRA occlusion. A randomized study comparing distal radial access and radial access is warranted to delineate the clinical benefits and disadvantages of this alternative route.
This study has several limitations. First, the arterial diameters might have been underestimated because vasodilators were not intended to be used before measurements were taken in the echocardiography laboratory. Second, the measured DRA diameters do not reflect normative values in the general population. However, patient-level data may be valuable for individuals scheduled to undergo invasive coronary procedures. Third, there was a discrepancy between the actual puncture site and the point of measurement. A standardized protocol for assessing the DRA diameter is warranted. Fourth, technical difficulties in identifying the true inner lumen may lead to vessel size overestimations and an increased risk of arterial occlusion.
In conclusion, here we provided the DRA reference diameters of Korean patients. Approximately 50% of patients had a DRA diameter < 2.3 mm. Female sex, a low BMI, and a low BSA remained significant predictors of a DRA diameter < 2.3 mm. Ultrasonographic evaluations with clinical assessments may guide the use of distal radial access.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



