 |
 |
| Korean J Intern Med > Volume 37(6); 2022 > Article |
|
Abstract
Musculoskeletal conditions are common in patients with diabetes. Several musculoskeletal disorders are viewed as chronic complications of diabetes because epidemiological studies have revealed high correlations between such complications and diabetes, but the pathophysiological links with diabetes remains unclear. Genetic predispositions, shared risk factors, microvascular impairments, progressive accumulation of advanced glycation end-products, and diabetic neuropathy may underlie the development of musculoskeletal disorders. Musculoskeletal complications of diabetics have received less attention than life-threatening microvascular or macrovascular complications. Here, we review several diabetic musculoskeletal complications with a focus on the clinical importance of early recognition and management, which would improve quality of life and physical function.
Diabetes mellitus is a chronic disease that imposes enormous socioeconomic burdens attributable to complications of various bodily systems. Diabetes affected 463 million people (9.3% of all people) in 2019 and will affect 700 million (10.9%) by 2045 [1]. In 2018, the prevalence of diabetes among Korean adults aged 30 years and older was 13.8% [2] and the 2019 healthcare costs were about 18 billion dollars [3]. The life expectancy of diabetics is increasing due to the development of novel antidiabetic drugs and medical techniques. This means that the burden of diseases reflecting the chronic complications of diabetes will also increase [4]. Macrovascular and microvascular complications directly threaten survival. Early recognition, prevention, and treatment of such complications have been prioritized. Epidemiologically, musculoskeletal disorders (MSDs) are recognized chronic complications [5] but have received less attention than the life-threatening vascular complications. Painful MSDs significantly compromise quality of life (QoL). However, such conditions are very treatable; early diagnosis and treatment of MSDs improve QoL [6]. Here, we review the relevant MSDs (Table 1).
It remains unclear why diabetes is associated with pathophysiological fibroproliferative complications in soft tissues. Many studies have found that elevated levels of advanced glycation end-products (AGEs) are associated with the microvascular complications of diabetes [7,8]. Abnormal AGE accumulation may trigger fibroproliferative complications [9]; proteins such as collagen that exhibit low biological turnover rates may be particularly susceptible to glycation [10]. Boivin et al. [11] showed histologically that the maximal tendon load, tensile stress, stiffness, and elasticity were low in an animal model of diabetes. Reddy et al. [12,13] reported that in rabbit tendons AGE cross-linking reduces sensitivity to collagenase and remodeling capacity, and increases stiffness. An inflammatory response can also trigger fibroproliferative complications in diabetics. Chondrocytes and tendon cell membranes host a specific AGE receptor [14]. Franke et al. [15] found that AGEs enhance transcription of the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) and upregulate the production of pro-inflammatory mediators including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). The levels of cytokines and vascular endothelial growth factors increase in the rotator cuffs of diabetics [16]. Increased levels of reactive oxygen species in hyperglycemic environments trigger inflammatory cascades and induce cell damage caused by various cytokines and growth factors [17].
It is clear that fibroproliferative and microvascular complications are linked; the latter should be examined if the former are present [18,19]. As is true of other diabetic complications, strict glycemic control may prevent the onset of such complications and/or delay progression but not completely reverse them [20]. No treatment that reverses pathological disease progression is yet available. Initial conservative treatments seek to control pain and limitations of motion. Surgery is possible if a response is lacking, and when an irreversible deformity develops.
Limited joint mobility syndrome (LJMS) is characterized by limited motion of the small hand and finger joints [21]. Stiff hands in patients with longstanding diabetes were first described by Lundbaek in 1957 [22]. The prevalence of LJMS is 8% to 58% in patients with type 1 diabetes mellitus (T1DM) and 25% to 76% in those with type 2 diabetes mellitus (T2DM); the rate in general populations is 1% to 20% [23]. The symptoms include painless stiffness, fixed flexion contracture, impaired fine movement, and decreased grip strength (both hands and fingers) [24]. The skin of the dorsum of the hand may become thick and waxy prior to motion limitation [25]. Helpful clinical features include the “prayer sign” and the “tabletop sign.” The prayer sign is present when the entire surface of the hand cannot be appressed when the palm and fingers contact the wrist during dorsiflexion (Fig. 1A). The tabletop sign is positive if the palmar surface does not fully touch the table, rather falling away when the palm contacts the tabletop at a right angle (Fig. 1B) [26]. Diseases such as osteoarthritis, rheumatoid arthritis, scleroderma, and systemic lupus erythematosus must be excluded [25]. LJMS can also develop in joints other than the fingers. For example, LJMS in the small joints of the foot may increase the risk for falls or diabetic foot ulcers [27,28]. Daily stretching exercises may help slow the progression of joint stiffness, and analgesics or corticosteroid injections may relieve pain or joint contracture [29,30]. Surgery may be necessary if such contracture or a deformity is severe [30].
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy caused by compression of the median nerve within the osteofibrous canal (the carpal tunnel) [31]. Diabetes is a major risk factor for CTS; the prevalence is much higher (14% to 30%) in diabetics than general populations (3.8%) [32,33]. A meta-analysis of 18 studies involving > 37 million individuals found that the pooled odds ratio (OR) for diabetic patients was 1.69 (95% confidence interval [CI], 1.45 to 1.96) [34]. In a recent cohort study using a National Diabetes Register containing data on approximately 1.1 million residents of Sweden, the prevalence ratios of CTS among patients with T1DM and T2DM compared to those without diabetes were 3.7–4.5 and 2.0–2.5, respectively [35]. Nerve compression triggers typical paresthesia of the thumb, index finger, middle finger, and the radial side of the ring finger, causing thenar muscle weakness and atrophy of the affected finger in severe cases (Fig. 2) [36]. Physical examinations that identify paresthesia in the median nerve include tapping on the median nerve of the wrist (the Tinel test), full palmar flexion of the wrist for more than 1 minute (the Phalen test), and raising the hand over the head for more than 1 minute (the hand elevation test). The symptoms may improve when the wrist is shaken or flicked (the flick sign). If CTS is clinically suspected, the condition can be confirmed electrophysiologically; the focal nerve conduction velocity is decreased at the entrapment site [36]. Recently, high-resolution ultrasound has been used to verify compression and morphological change in the median nerve [36]. Corticosteroid injections help relieve CTS symptoms [37]. Surgical decompression is the treatment of choice for patients evidencing severe nerve damage in nerve conduction studies if the etiology is not reversible [38]. Diabetic patients are 4- to 14-fold more likely to require surgery than general populations [39].
Stenosing tenosynovitis (commonly termed trigger finger) is caused by inflammation attributable to repeated friction between the flexor tendon and the sheaths that form the tunnel affording mechanical stability to that tendon [40]. Inflammation causes the tendon and sheaths to swell and form nodules. The flexor tendon becomes trapped in the tunnel, and the finger joint is then locked in flexion (Fig. 3A) [41]. The prevalence in diabetic patients is 5% to 20%, much higher than the 1% to 2% in general populations [42]. Unlike in such populations, trigger finger in diabetics is more common in women and is characterized by bilateral and multiple finger involvement [43]. Extensor stretching exercises can help prevent recurrence [44]. Immobilization is preferred in the acute phase, and local corticosteroid injections can be considered in severe cases [45]. If conservative treatments fail, open surgery releases the A1 pulley [45].
Dupuytren’s contracture (DC) is a progressive condition; nodules or contractures form in the palmar fascia, caused by fibrosis. The incidence of DC in diabetic patients is about 16% to 42% [46] and is related to the duration of diabetes, old age, the male sex, smoking, and alcohol consumption [47]. Initially, nodules develop near the metacarpophalangeal joint on the palmar side, creating a fibrous band with the finger, resulting in flexion contracture and limited movement (Fig. 3B) [48]. DC principally affects the fourth and fifth fingers (unlike trigger finger, which affects primarily the thumb and the index and middle fingers) [48]. Local corticosteroid injections may be considered if tenderness is evident or the nodule grows [46]. Intralesional, clostridial collagenase injections relieve the contractures and improve joint movement [49]. Surgery may be considered if joint movement is impaired by progressive flexion contracture [46]. Recurrence after surgery is more frequent in diabetic patients [50].
Adhesive capsulitis (AC), also termed “frozen shoulder” (by the American Academy of Orthopedic Surgeons), is “a condition of varying severity characterized by the gradual development of global limitation of active and passive shoulder motion where radiographic findings other than osteopenia are absent” [51]. AC is triggered by inflammation that in turn causes fibrosis of the glenohumeral joint capsule and adhesion of surrounding structures [52]. Diabetes is a significant risk factor for AC; the prevalence in diabetics is 19% to 29%, much higher than the approximately 5% in general populations [53,54]. Old age, long diabetes duration, and poor glycemic control increase its prevalence, and the prognosis is poorer in diabetics than general populations [53,55]. It is accompanied by progressive stiffness and significant restriction of the range of motion [56]. Pain or a limited range of motion develop during sudden movements such as shoulder external rotation or abduction [57]. Diagnosis is based primarily on clinical symptoms and physical examination. As the incidence of rotator cuff tendinopathy is also approximately 1.5-fold higher in diabetics than general populations, this condition should be considered during differential diagnosis of AC [58,59]. Non-contrast, shoulder magnetic resonance imaging (MRI) aids the differential diagnosis [60]. Most cases of AC resolve over time; physiotherapy and oral analgesics may accelerate symptom relief [61]. Local corticosteroid injections and hydrodilatation are useful adjunctive treatments [61,62]. Surgery may be required if conservative treatments elicit no response [61]. Diabetics evidence more severe symptoms than general populations and require early and frequent intensive management [57].
Neuropathic arthropathy, also termed Charcot arthropathy, is a progressive and destructive disease of the joints and adjacent bony structures caused by loss of sensation [63]. Charcot described the prototype of the disorder in the context of the tabes dorsalis [64]. Similar changes occur in patients with diabetic neuropathy, which is then termed diabetic neuroarthropathy (DN). Peripheral or autonomic neuropathies (microvascular complications of diabetes) can trigger mechanical or vascular joint changes; bone metabolic abnormalities seem to be associated with DN development [65]. The prevalence of DN is 0.1% to 7.5% in diabetic patients but 29% to 35% in those with diabetic peripheral neuropathy [66]. DN principally affects the foot and ankle joints; the symptoms include painless joint swelling, warmth when touched, instability, and deformity (Fig. 4) [65]. The physician should suspect DN when unilateral warmth, swelling, and erythema develop in a patient with diabetes of long duration and diabetic neuropathy. Infections (cellulitis, septic arthritis, or osteomyelitis), gout, osteoarthritis, and rheumatoid arthritis should be excluded [67]. Structural changes in the feet change weight-bearing and then trigger local trauma such as diabetic foot ulcers. The DN staging system of the modified Eichenholtz classification aids management (Table 2) [68]. Plain radiography may reveal the typical (stage-specific) findings of DN and MRI assists the differential diagnosis. In the acute phase, the most critical intervention is avoidance of foot weight-bearing by applying a cast or the use of crutches or a wheelchair [69]. Prevention of diabetic foot ulcers and infections is essential in patients exhibiting chronic and severe joint damage [69]. Surgical treatment may be considered if plantar stability cannot be maintained using special footwear or an orthosis to counter the foot deformity. However, surgery is best avoided [70].
Gout is an inflammatory form of arthritis in which monosodium urate crystals are deposited in the joints because of hyperuricemia [71]. Gout and diabetes interact in that each condition increases the incidence of the other, reflecting the correlation between hyperuricemia and insulin-resistance [72]. Although gout is a well-known major risk factor for diabetes [65], whether diabetes increases the risk for gout remains controversial. In a large, 3-year, community-based observational study, gout developed in 16.0% of patients with T2DM and 14.2% of those with prediabetes; the figures were significantly higher than the 2.7% of the general population [73]. In another prospective observational study, the relative risk for gout development in diabetics was as low as 0.77 (95% CI, 0.60 to 0.97) [74]. It may be that diabetes does not directly increase the risk for gout. Rather, the two conditions co-occur because they share risk factors or clinical features related to metabolic syndrome [75]. Gout exhibits two phases: recurrent acute inflammatory arthritis (Fig. 5) and chronic tophaceous gout [71]. During acute episodes, nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or colchicine are used to reduce acute inflammation. Patients evidencing recurrent acute attacks, gouty tophi, urinary stones, and radiologically confirmed damage are prescribed long-term urate-reducing agents such as probenecid, allopurinol, or febuxostat [76].
Osteoporosis is a skeletal disorder; bone strength is compromised by a decreased bone mass and microarchitectural damage, increasing the fracture risk [77]. Most epidemiological studies have reported increased risk for fragility fractures in diabetics. Bone mineral density (BMD) decreases in T1DM patients but increases in those with T2DM; however, the fragility fracture risks are enhanced in both T1DM and T2DM patients [78]. The fact that an increased BMD is nonetheless associated with a higher fracture risk in T2DM patients renders risk assessment and fracture prevention challenging. A recent meta-analysis of observational studies found that the relative risk for fracture in young and middle-aged adults with T1DM is 1.88 (95% CI, 1.52 to 2.32) [79] and that in adults with T2DM it is 1.31 (95% CI, 1.17 to 1.46) [80]. In the Women’s Health Initiative Observational Study, patients with T2DM exhibited a higher BMD, but the relative risk for any fracture was 1.20 (95% CI, 1.11 to 1.30) even after controlling for age, weight, fall frequency, tobacco and alcohol use, and exercise [81].
Any effect of glycemic control on the fracture risk remains controversial. Although strict glycemic control did not reduce that risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study [82], a large community-based observational study showed that the fracture risk was 24% to 63% higher in patients with poor glycemic control compared to those with low hemoglobin A1c (HbA1c) levels [83,84]. The duration of diabetes was also a risk factor. The relative risk for hip fracture in patients who were diabetic for more than (vs. less than) 10 years was 1.19 (95% CI, 1.13 to 1.25) [85]. Diabetes-related complications increase the risk for fracture. In a study that used data from a Danish registry, the fracture risk was higher in patients with T2DM accompanied by diabetic retinopathy (OR, 2.08; 95% CI, 1.80 to 2.41), nephropathy (OR, 2.02; 95% CI, 1.65 to 2.47), neuropathy (OR, 1.91; 95% CI, 1.64 to 2.21), or macrovascular complications (OR, 1.92; 95% CI, 1.61 to 2.28), compared to those with no complications (OR, 1.43; 95% CI, 1.35 to 1.50) [86].
The pathophysiology of bone fragility in diabetics is complicated. Most patients with T1DM exhibit complete β-cell failure and low levels of IGF-1 that reduce the peak bone mass when young because osteoblast function is impaired during growth [87]. Pro-inflammatory cytokines, adipokines, glucotoxicity, and AGEs inhibit Wnt signaling. In T2DM patients, skeletal microvascular changes are thought to render osteocyte function and collagen synthesis abnormal, and to decrease bone turnover [88]. Complications such as neuropathy, balance disturbances, sarcopenia, vision impairment, and hypoglycemic events increase the risk for falls and consequent fractures [89].
Of the various glucose-lowering medications, some increase the fracture risk. For example, thiazolidinediones (TZDs) activate peroxisome proliferator-activated receptors and impair osteoblastogenesis, decreasing the BMD and increasing fracture risk. A meta-analysis of 10 randomized controlled trials and two observational studies reported an increased risk for fracture in women treated with TZDs (OR, 2.23; 95% CI, 1.65 to 3.01) [90]. In the Canagliflozin Cardiovascular Assessment Study (CANVAS), of the sodium-glucose cotransporter-2 (SGLT-2) inhibitors, canagliflozin caused hip bone loss and increased the hip fracture risk (hazard ratio [HR], 1.26; 95% CI, 1.04 to 1.52) [91]. Conversely, the incidence of fractures did not increase in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial (HR, 0.98; 95% CI, 0.70 to 1.37). In other studies, dapagliflozin and empagliflozin did not increase fracture incidence (dapagliflozin [HR, 1.04; 95% CI, 0.91 to 1.18]; empagliflozin: 3.9% and 3.8% in the placebo and empagliflozin groups, respectively) [92,93]. In a recent meta-analysis of 30 randomized controlled trials, the pooled OR of bone fractures was 0.86 (95% CI, 0.70 to 1.06) [94].
There is no specific guideline for osteoporosis management in diabetics. The pharmacological treatments and fall-prevention strategies for diabetics at high risk for fracture are the same as those for osteoporotic patients without diabetes. However, T2DM patients are at increased fracture risk even if the BMD does not decrease. Thus, novel strategies for preventing fragile fractures in patients with T2DM are required; these should differ from those for osteoporosis patients without T2DM but a low BMD. More active fracture risk assessment and earlier medical intervention should be considered in patients with diabetes of long duration or microvascular complications, and those at higher risk for falls because of recurrent hypoglycemia or neurological abnormalities [95].
Diffuse idiopathic skeletal hyperostosis (DISH) is characterized by calcification and ossification of the anterolateral ligaments of the spine [96]. The etiology of DISH remains unknown, but several studies have revealed epidemiological correlations with diabetes. The prevalence of DISH in diabetics is 5% to 50%, higher than in general populations [97]. DISH is most common in the thoracic spine and is diagnosed when calcification of four or more consecutive vertebral ligaments is evident [98]. However, narrowing of the intervertebral space, inflammation of the sacroiliac joint, and signs of degenerative disease or inflammatory spondyloarthropathy must be excluded [99]. Most patients progress slowly and are asymptomatic, but symptoms such as spinal stiffness, lower back pain, limited movement, or compression of surrounding organs may develop [100]. Physiotherapy or NSAIDs may be used to treat pain and stiffness [100]. If there is no response, local corticosteroid injections may help [101]. Surgical treatment may be required if myelopathy, neuropathy, or dysphagia develop because of compression of surrounding organs [101].
Sarcopenia is characterized by a progressive deterioration of muscle mass and function with aging. Several medical conditions may accelerate progression, which is closely related to insulin-resistance, impaired fasting glucose levels, and diabetes [102]. The Health, Aging and Body Composition Study showed that deterioration of muscle mass and muscle strength accelerated in elderly diabetics (compared to non-diabetics) exhibiting poor glycemic control (HbA1c > 8%) for at least 6 years [103]. In another study, elderly women with T2DM exhibited about twice as much muscle mass loss than non-diabetic women after 6 years of follow-up [104]. In a study of 414 Koreans more than 65 years old, sarcopenia development was two to four times greater in a T2DM group than a control group [105]. In the Korean Frailty and Aging Cohort Study that enrolled 2,403 elderly women aged 70 to 84 years, those exhibiting insulin-resistance or diabetes (14.7% and 8.5%, respectively) evidenced twice as much sarcopenia than others [106]. Conversely, sarcopenia is a risk factor for diabetes [107]. The U.S. National Health and Nutrition Examination Survey (NHANES) III data revealed that the higher the muscle mass, the lower the extent of insulin-resistance and the lower the risk for diabetes regardless of obesity status [108,109]. The Korea National Health and Nutrition Examination Survey IV (KNHANES IV) found a significant association between insulin-resistance and sarcopenia [110]. Another Korean study on 493 healthy adults (180 men and 313 women) reported that the homeostatic model assessment of insulin-resistance was negatively correlated with muscle mass [111]. The possible pathophysiological mechanisms of sarcopenia in older patients with diabetes include decreased anabolic activity attributable to increased insulin-resistance [112], upregulation of inflammatory cytokines such as TNF-α or IL-6, and/or mitochondrial dysfunction [113].
Diabetic muscle infarction (DMI) is a spontaneous ischemic necrosis of skeletal muscle in the absence of an arterial thromboembolism or an atherosclerotic occlusion of a large artery [114]. It is a rare complication of patients with both longstanding diabetes and multiple microvascular or macrovascular complications [115]. It affects principally the calf and thigh muscles, accompanied by muscle pain and swelling [116]. Nonspecific increases in the levels of muscle enzymes such as creatine kinase, the leukocyte number, the C-reactive protein level, and the erythrocyte sedimentation rate may be noted [116]. When diagnosing DMI, acute arterial occlusion, infection, and a malignant tumor must be excluded. Plain radiography, ultrasound, and MRI may aid diagnosis, but a muscle biopsy may be required [117]. Low-dose aspirin is the preferred antiplatelet treatment (this prevents ischemia progression), but clopidogrel is an alternative; NSAIDs allow of short-term pain relief and other pain relievers can be prescribed if aspirin side effects are of concern [116].
MSDs in patients with diabetes are more common than in general populations, although the disorders are not confined to diabetics. The disorders significantly impact QoL and the activities of daily living. Early evaluation and management of musculoskeletal problems in diabetics reduce pain, improve QoL, and minimize morbidity and mortality. It is important to consider the clinical characteristics, diagnoses, and treatments of musculoskeletal complications when comprehensively managing diabetes.
Figure 1
Clinical signs of limited joint mobility syndrome. The “prayer sign” (A) and the “tabletop sign” (B) in a middle-aged woman with long-term type 2 diabetes. The prayer sign reflects an inability to appress the flattened palms (as when praying). The tabletop sign reflects an inability to place the complete palm on the surface of a table with the wrist at a right angle.
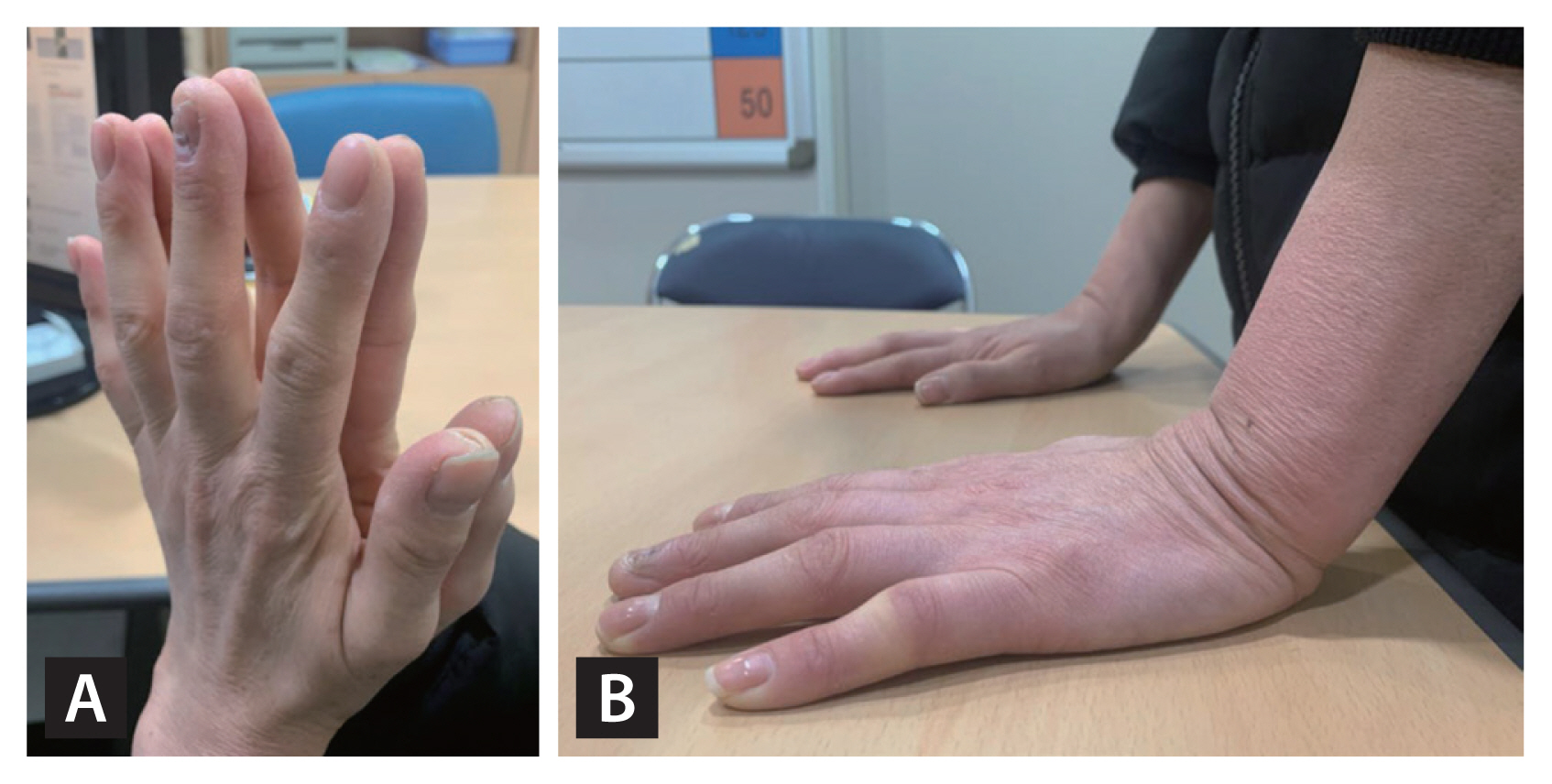
Figure 2
Thenar muscle atrophy in a patient with carpal tunnel syndrome. The yellow arrow indicates thenar muscle atrophy reflecting the severe and prolonged median nerve compression of carpal tunnel syndrome.
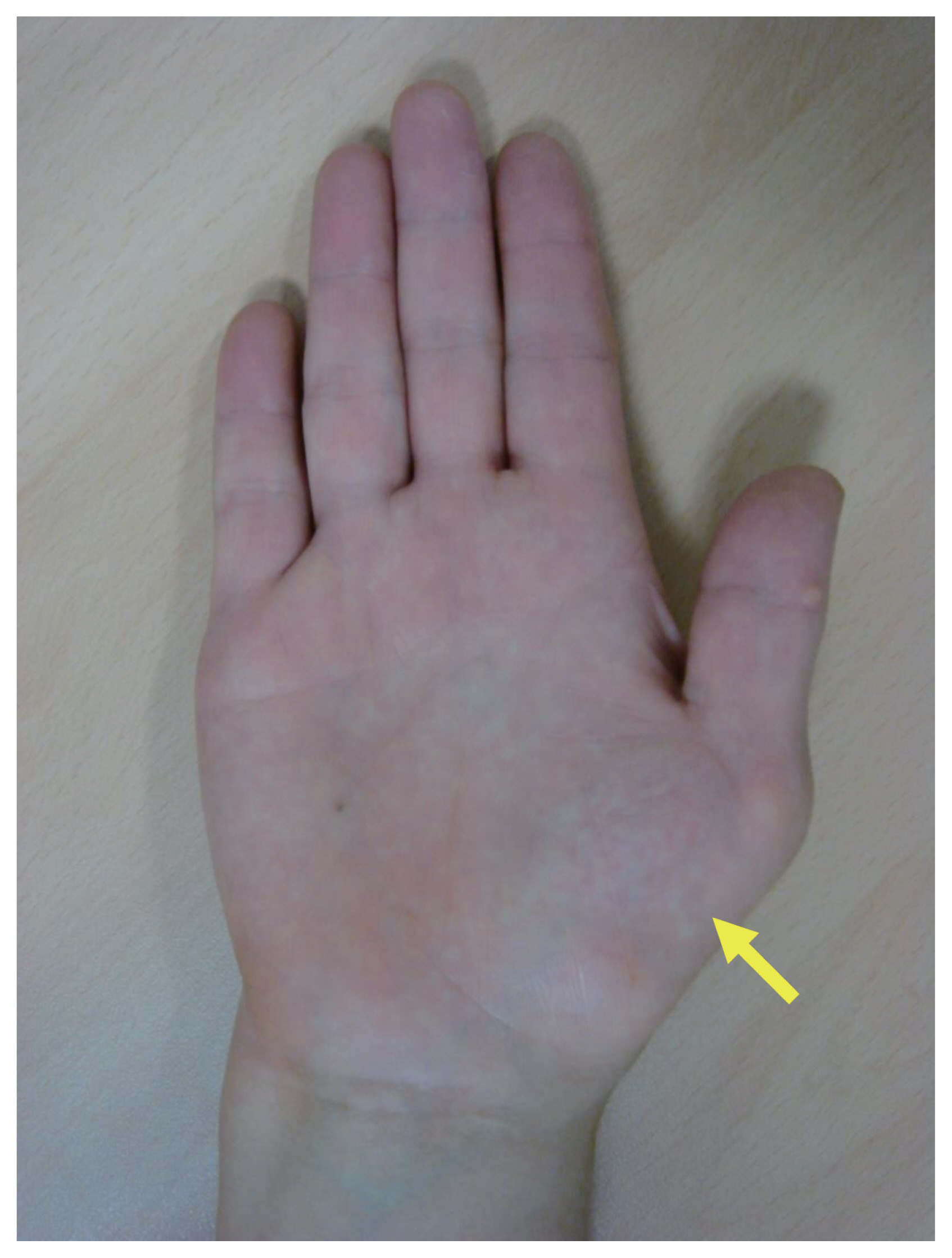
Figure 3
Flexion contractures in patients with stenosing tenosynovitis (trigger finger) (A) and Dupuytren’s contracture (B). Yellow arrows indicate fingers locked in the flexed position in patients with severe stenosing tenosynovitis (trigger finger) (A) and Dupuytren’s contracture (B); it was impossible to straighten the fingers. The white arrow in (B) indicates thickened finger tissue.
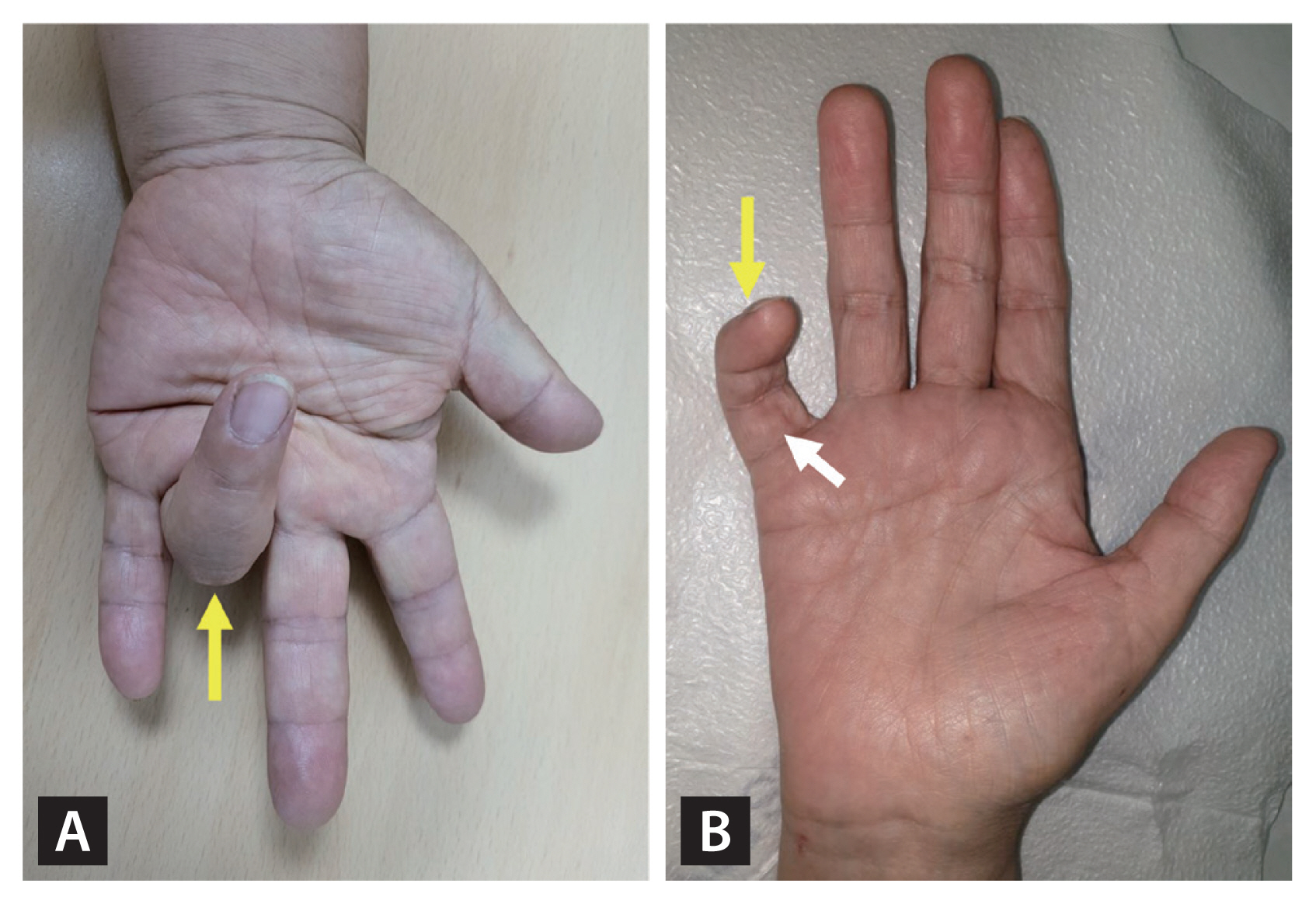
Figure 4
Foot deformities in a patient with diabetic neuroarthropathy (Charcot arthropathy). Patients with diabetic neuropathy may exhibit various foot deformities depending on the locations of the microfractures. As shown in this photograph, the toes may be curved to the medial side (yellow arrow) or be clawlike (white arrows).

Figure 5
Acute inflammatory arthritis in a patient with gouty arthritis. The yellow arrow indicates acute inflammation with swelling of, and redness at, the base of the left great toe in a patient with gouty arthritis.
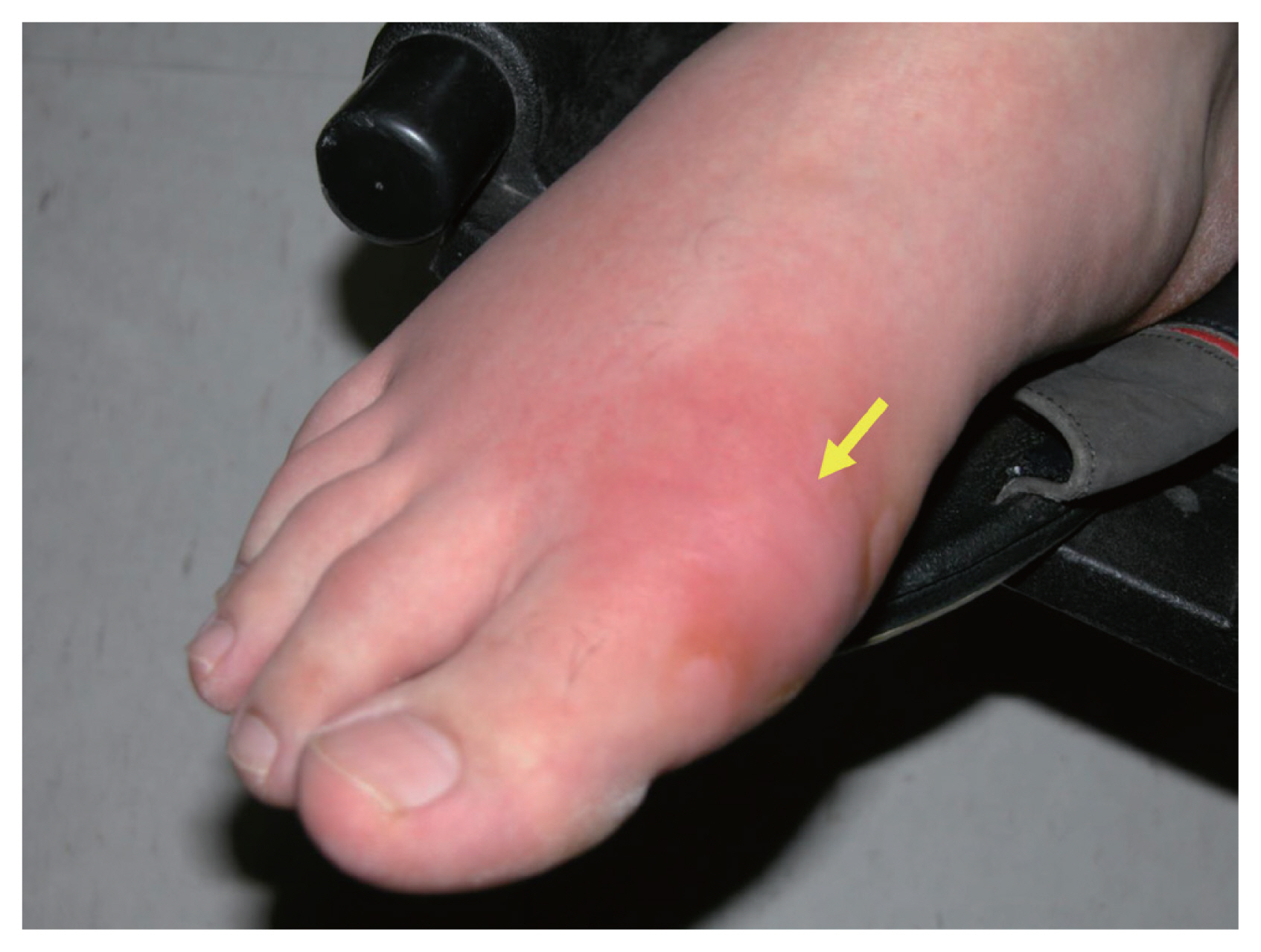
Table 1
Common musculoskeletal complications in patients with diabetes mellitus
Table 2
The staging system for Charcot arthropathy that uses the modified Eichenholtz classification
Adapted from Rosenbaum et al. [68], with permission from Wolters Kluwer Health, Inc.
REFERENCES
1. International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels (BE): International Diabetes Federation, 2019.
2. Jung CH, Son JW, Kang S, et al. Diabetes fact sheets in Korea, 2020: an appraisal of current status. Diabetes Metab J 2021;45:1–10.




3. Oh SH, Ku H, Park KS. Prevalence and socioeconomic burden of diabetes mellitus in South Korean adults: a population-based study using administrative data. BMC Public Health 2021;21:548.




4. Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia 2019;62:3–16.



5. Pandey A, Usman K, Reddy H, Gutch M, Jain N, Qidwai S. Prevalence of hand disorders in type 2 diabetes mellitus and its correlation with microvascular complications. Ann Med Health Sci Res 2013;3:349–354.



6. Adriaanse MC, Drewes HW, van der Heide I, Struijs JN, Baan CA. The impact of comorbid chronic conditions on quality of life in type 2 diabetes patients. Qual Life Res 2016;25:175–182.




7. Monnier VM, Sell DR, Gao X, et al. Plasma advanced glycation end products and the subsequent risk of microvascular complications in type 1 diabetes in the DCCT/EDIC. BMJ Open Diabetes Res Care 2022;10:e002667.



8. Khalid M, Petroianu G, Adem A. Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules 2022;12:542.



9. Holte KB, Juel NG, Brox JI, et al. Hand, shoulder and back stiffness in long-term type 1 diabetes; cross-sectional association with skin collagen advanced glycation end-products: the Dialong study. J Diabetes Complications 2017;31:1408–1414.


10. Avery NC, Bailey AJ. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol Biol (Paris) 2006;54:387–395.


11. Boivin GP, Elenes EY, Schultze AK, Chodavarapu H, Hunter SA, Elased KM. Biomechanical properties and histology of db/db diabetic mouse Achilles tendon. Muscles Ligaments Tendons J 2014;4:280–284.



12. Reddy GK, Stehno-Bittel L, Enwemeka CS. Glycation-induced matrix stability in the rabbit Achilles tendon. Arch Biochem Biophys 2002;399:174–180.


13. Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp Diabesity Res 2004;5:143–153.




14. Valencia JV, Weldon SC, Quinn D, et al. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem 2004;324:68–78.


15. Franke S, Sommer M, Ruster C, et al. Advanced glycation end products induce cell cycle arrest and proinflammatory changes in osteoarthritic fibroblast-like synovial cells. Arthritis Res Ther 2009;11:R136.



16. Handa A, Gotoh M, Hamada K, et al. Vascular endothelial growth factor 121 and 165 in the subacromial bursa are involved in shoulder joint contracture in type II diabetics with rotator cuff disease. J Orthop Res 2003;21:1138–1144.


17. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625.

18. Larkin ME, Barnie A, Braffett BH, et al. Musculoskeletal complications in type 1 diabetes. Diabetes Care 2014;37:1863–1869.




19. Arkkila PE, Kantola IM, Viikari JS. Limited joint mobility in non-insulin-dependent diabetic (NIDDM) patients: correlation to control of diabetes, atherosclerotic vascular disease, and other diabetic complications. J Diabetes Complications 1997;11:208–217.


20. Silverstein JH, Gordon G, Pollock BH, Rosenbloom AL. Longterm glycemic control influences the onset of limited joint mobility in type 1 diabetes. J Pediatr 1998;132:944–947.


21. Kapoor A, Sibbitt WL Jr. Contractures in diabetes mellitus: the syndrome of limited joint mobility. Semin Arthritis Rheum 1989;18:168–180.


23. Sozen T, Basaran NC, Tinazli M, Ozisik L. Musculoskeletal problems in diabetes mellitus. Eur J Rheumatol 2018;5:258–265.



24. Gerrits EG, Landman GW, Nijenhuis-Rosien L, Bilo HJ. Limited joint mobility syndrome in diabetes mellitus: a minireview. World J Diabetes 2015;6:1108–1112.



25. Hill NE, Roscoe D, Stacey MJ, Chew S. Cheiroarthropathy and tendinopathy in diabetes. Diabet Med 2019;36:939–947.



26. Al-Homood IA. Rheumatic conditions in patients with diabetes mellitus. Clin Rheumatol 2013;32:527–533.



27. Lopez-Martin I, Benito Ortiz L, Rodriguez-Borlado B, Cano Langreo M, Garcia-Martinez FJ, Martin Rodriguez MF. Association between limited joint mobility syndrome and risk of accidental falls in diabetic patients. Semergen 2015;41:70–75.

28. Zimny S, Schatz H, Pfohl M. The role of limited joint mobility in diabetic patients with an at-risk foot. Diabetes Care 2004;27:942–946.



29. Francia P, Gulisano M, Anichini R, Seghieri G. Diabetic foot and exercise therapy: step by step the role of rigid posture and biomechanics treatment. Curr Diabetes Rev 2014;10:86–99.



30. Abate M, Schiavone C, Salini V, Andia I. Management of limited joint mobility in diabetic patients. Diabetes Metab Syndr Obes 2013;6:197–207.



31. Olney RK. Carpal tunnel syndrome: complex issues with a “simple” condition. Neurology 2001;56:1431–1432.


32. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999;282:153–158.


33. Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care 2002;25:565–569.



34. Pourmemari MH, Shiri R. Diabetes as a risk factor for carpal tunnel syndrome: a systematic review and meta-analysis. Diabet Med 2016;33:10–16.


35. Rydberg M, Zimmerman M, Gottsater A, Svensson AM, Eeg-Olofsson K, Dahlin LB. Diabetic hand: prevalence and incidence of diabetic hand problems using data from 1.1 million inhabitants in southern Sweden. BMJ Open Diabetes Res Care 2022;10:e002614.



36. Padua L, Coraci D, Erra C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol 2016;15:1273–1284.


37. Atroshi I, Flondell M, Hofer M, Ranstam J. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med 2013;159:309–317.


38. Shi Q, MacDermid JC. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome?: a systematic review. J Orthop Surg Res 2011;6:17.



39. Makepeace A, Davis WA, Bruce DG, Davis TM. Incidence and determinants of carpal tunnel decompression surgery in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care 2008;31:498–500.

40. Jeanmonod R, Harberger S, Waseem M. Trigger finger. StatPearls Treasure Island (FL): StatPearls Publishing, 2022. [cited 2022 Aug 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459310
.
42. Vance MC, Tucker JJ, Harness NG. The association of hemoglobin A1c with the prevalence of stenosing flexor tenosynovitis. J Hand Surg Am 2012;37:1765–1769.


43. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am 2008;33:771–775.


44. Ferrara PE, Codazza S, Maccauro G, Zirio G, Ferriero G, Ronconi G. Physical therapies for the conservative treatment of the trigger finger: a narrative review. Orthop Rev (Pavia) 2020;12(Suppl 1):8680.




45. Kuczmarski AS, Harris AP, Gil JA, Weiss AC. Management of diabetic trigger finger. J Hand Surg Am 2019;44:150–153.


46. Noble J, Heathcote JG, Cohen H. Diabetes mellitus in the aetiology of Dupuytren’s disease. J Bone Joint Surg Br 1984;66:322–325.



47. Hart MG, Hooper G. Clinical associations of Dupuytren’s disease. Postgrad Med J 2005;81:425–428.



48. Trojian TH, Chu SM. Dupuytren’s disease: diagnosis and treatment. Am Fam Physician 2007;76:86–89.

49. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009;361:968–979.


50. Norotte G, Apoil A, Travers V. A ten years follow-up of the results of surgery for Dupuytren’s disease: a study of fifty-eight cases. Ann Chir Main 1988;7:277–281.

51. St Angelo JM, Fabiano SE. Adhesive capsulitis. StatPearls Treasure Island (FL): StatPearls Publishing, 2022. [cited 2022 Aug 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532955
.
52. Baslund B, Thomsen BS, Jensen EM. Frozen shoulder: current concepts. Scand J Rheumatol 1990;19:321–325.


53. Balci N, Balci MK, Tuzuner S. Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes mellitus: association with diabetic complications. J Diabetes Complications 1999;13:135–140.

54. Huang YP, Fann CY, Chiu YH, et al. Association of diabetes mellitus with the risk of developing adhesive capsulitis of the shoulder: a longitudinal population-based follow up study. Arthritis Care Res (Hoboken) 2013;65:1197–1202.


55. Gundtoft PH, Attrup ML, Kristensen AK, Vobbe JW, Sorensen L, Holmich P. Diabetes mellitus affects the prognosis of frozen shoulder. Dan Med J 2020;67:A02200071.

56. Lewis J. Frozen shoulder contracture syndrome: aetiology, diagnosis and management. Man Ther 2015;20:2–9.


57. Whelton C, Peach CA. Review of diabetic frozen shoulder. Eur J Orthop Surg Traumatol 2018;28:363–371.



58. Titchener AG, White JJ, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case-control study. J Shoulder Elbow Surg 2014;23:1282–1288.


59. Lin TT, Lin CH, Chang CL, Chi CH, Chang ST, Sheu WH. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med 2015;43:2126–2132.



60. Chi AS, Kim J, Long SS, Morrison WB, Zoga AC. Non-contrast MRI diagnosis of adhesive capsulitis of the shoulder. Clin Imaging 2017;44:46–50.


61. Robinson CM, Seah KT, Chee YH, Hindle P, Murray IR. Frozen shoulder. J Bone Joint Surg Br 2012;94:1–9.


62. Cho JH. Updates on the treatment of adhesive capsulitis with hydraulic distension. Yeungnam Univ J Med 2021;38:19–26.




65. Trieb K. The Charcot foot: pathophysiology, diagnosis and classification. Bone Joint J 2016;98-B:1155–1159.

66. Schoots IG, Slim FJ, Busch-Westbroek TE, Maas M. Neuro-osteoarthropathy of the foot-radiologist: friend or foe? Semin Musculoskelet Radiol 2010;14:365–376.


67. Marmolejo VS, Arnold JF, Ponticello M, Anderson CA. Charcot foot: clinical clues, diagnostic strategies, and treatment principles. Am Fam Physician 2018;97:594–599.

68. Rosenbaum AJ, DiPreta JA. Classifications in brief: Eichenholtz classification of Charcot arthropathy. Clin Orthop Relat Res 2015;473:1168–1171.



69. Schmidt BM, Holmes CM. Updates on diabetic foot and Charcot osteopathic arthropathy. Curr Diab Rep 2018;18:74.



70. Guven MF, Karabiber A, Kaynak G, Ogut T. Conservative and surgical treatment of the chronic Charcot foot and ankle. Diabet Foot Ankle 2013;Aug. 2. 4:21177.



71. Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep 2014;16:400.




72. Vuorinen-Markkola H, Yki-Jarvinen H. Hyperuricemia and insulin resistance. J Clin Endocrinol Metab 1994;78:25–29.


73. Liu Q, Gamble G, Pickering K, Morton S, Dalbeth N. Prevalence and clinical factors associated with gout in patients with diabetes and prediabetes. Rheumatology (Oxford) 2012;51:757–759.


74. Pan A, Teng GG, Yuan JM, Koh WP. Bidirectional association between diabetes and gout: the Singapore Chinese Health Study. Sci Rep 2016;6:25766.




75. Lai HM, Chen CJ, Su BY, et al. Gout and type 2 diabetes have a mutual inter-dependent effect on genetic risk factors and higher incidences. Rheumatology (Oxford) 2012;51:715–720.


76. Engel B, Just J, Bleckwenn M, Weckbecker K. Treatment options for gout. Dtsch Arztebl Int 2017;114:215–222.



77. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–795.


78. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta-analysis. Osteoporos Int 2007;18:427–444.



79. Thong EP, Herath M, Weber DR, et al. Fracture risk in young and middle-aged adults with type 1 diabetes mellitus: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2018;89:314–323.




80. Ni Y, Fan D. Diabetes mellitus is a risk factor for low bone mass-related fractures: a meta-analysis of cohort studies. Medicine (Baltimore) 2017;96:e8811.


81. Bonds DE, Larson JC, Schwartz AV, et al. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab 2006;91:3404–3410.


82. Schwartz AV, Margolis KL, Sellmeyer DE, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care 2012;35:1525–1531.




83. Li CI, Liu CS, Lin WY, et al. Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan Diabetes Cohort Study. J Bone Miner Res 2015;30:1338–1346.


84. Oei L, Zillikens MC, Dehghan A, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care 2013;36:1619–1628.


85. Moayeri A, Mohamadpour M, Mousavi SF, Shirzadpour E, Mohamadpour S, Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta-analysis. Ther Clin Risk Manag 2017;13:455–468.




86. Vestergaard P, Rejnmark L, Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int 2009;84:45–55.



87. Hough FS, Pierroz DD, Cooper C, Ferrari SL, IOF CSA Bone and Diabetes Working Group. Mechanisms in endocrinology: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol 2016;174:R127–R138.


88. Napoli N, Chandran M, Pierroz DD, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol 2017;13:208–219.



89. Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002;25:1749–1754.

90. Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 2009;180:32–39.



91. Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2016;101:157–166.



92. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357.


93. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128.


94. Cheng L, Li YY, Hu W, et al. Risk of bone fracture associated with sodium-glucose cotransporter-2 inhibitor treatment: a meta-analysis of randomized controlled trials. Diabetes Metab 2019;45:436–445.


95. Ferrari SL, Abrahamsen B, Napoli N, et al. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 2018;29:2585–2596.




97. Pillai S, Littlejohn G. Metabolic factors in diffuse idiopathic skeletal hyperostosis: a review of clinical data. Open Rheumatol J 2014;8:116–128.




98. Vaishya R, Vijay V, Nwagbara IC, Agarwal AK. Diffuse idiopathic skeletal hyperostosis (DISH): a common but less known cause of back pain. J Clin Orthop Trauma 2017;8:191–196.



99. Angelopoulou F, Kraniotis P, Daoussis D. DISH vs spondyloar-thritides. Mediterr J Rheumatol 2020;31:81–83.



100. Mader R, Verlaan JJ, Eshed I, et al. Diffuse idiopathic skeletal hyperostosis (DISH): where we are now and where to go next. RMD Open 2017;3:e000472.



101. Mader R, Verlaan JJ, Buskila D. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol 2013;9:741–750.



102. Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab 2005;31(Spec 2):5S20–5S26.


103. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006;55:1813–1818.

104. Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997.




105. Kim KS, Park KS, Kim MJ, Kim SK, Cho YW, Park SW. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr Gerontol Int 2014;14:Suppl 1. 115–121.


106. Kang S, Oh TJ, Cho BL, et al. Sex differences in sarcopenia and frailty among community-dwelling Korean older adults with diabetes: the Korean Frailty and Aging Cohort Study. J Diabetes Investig 2021;12:155–164.




107. Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes 2019;12:1057–1072.


108. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One 2010;5:e10805.



109. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes: findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 2011;96:2898–2903.


110. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr J 2014;61:61–70.


111. Kim TN, Park MS, Lim KI, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean Sarcopenic Obesity Study. Clin Endocrinol (Oxf) 2013;78:525–532.


112. Morais JA, Jacob KW, Chevalier S. Effects of aging and insulin resistant states on protein anabolic responses in older adults. Exp Gerontol 2018;108:262–268.


113. Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res 2017;22:25.




114. Yong TY, Khow KS. Diabetic muscle infarction in end-stage renal disease: a scoping review on epidemiology, diagnosis and treatment. World J Nephrol 2018;7:58–64.



115. Trujillo-Santos AJ. Diabetic muscle infarction: an underdiagnosed complication of long-standing diabetes. Diabetes Care 2003;26:211–215.

-
METRICS

- Related articles
-
Metabolic musculoskeletal disorders in patients with inflammatory bowel disease2025 March;40(2)
Korean treatment recommendations for patients with axial spondyloarthritis2024 January;39(1)
Long-term renal outcomes of patients with non-proliferative lupus nephritis2023 September;38(5)
Korean treatment recommendations for patients with axial spondyloarthritis2023 September;38(5)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


