INTRODUCTION
LofflerŌĆÖs syndrome (simple pulmonary eosinophilia) are characterized by minimal pulmonary symptoms, transient pulmonary infiltrates that tend to be patchy, and mild peripheral blood eosinophilia. Various parasitic infestations are known to sometimes cause eosinophilic pulmonary infiltrations1). Although clonorchiasis is a common hepatobiliary disease in Korea that produces marked eosinophilia in the peripheral circulation, its clinical presentation rarely includes pulmonary infiltrates2). In the MEDLINE search of English language articles published from 1960 through 2003, only two cases of eosinophilic pulmonary infiltrations associated with Clonorchis sinensis were found3, 4). We report a case of LofflerŌĆÖs syndrome associated with Clonorchis sinensis infestation in Korea.
CASE REPORT
A 54-year-old man presented with a several-day history of erythematous rashes on his extremities. He also had a history of ingesting raw freshwater fish, but not of bronchial asthma or other medical side effects. He had visited a community clinic where a chest radiograph showed a solitary pulmonary nodule in the right upper lobe (Figure 1). This nodule was not present on a chest radiograph taken 4 years before. The rashes subsided following treatment with an antihistamine and a non-steroidal anti-infilammatory agent. The patientŌĆÖs physical examination was unremarkable. On admission, his leukocyte count was 11,250 cells/╬╝L with 38% neutrophils, 23% lymphocytes, 4% monocytes, and 35% eosinophils. Other hematologic parameters were 13.5 g/dL hemoglobin, 7 mm/hr ESR, 12.5 sec (control=11.5 sec) PT, and 28 sec (INR=1.0) PTT. Urinalysis, blood chemistry, pulmonary function test, and electrocardiography were normal. Anti-Hepatitis B surface antigen was negative, but anti-Hepatitis B surface antibody was positive. All stains and cultures of sputum for bacteria and mycobacteria were negative. There were no malignant cells in sputum cytology, but many eosinophils were present. Stool examination for helminths revealed many Clonorchis sinensis ova; stool ova count was 200 eggs per gram. A skin test for Paragonimus westermani was negative, but one for Clonorchis sinensis was strongly positive. Total serum IgE was 1020 IU/mL and Clonorchis-specific IgG antibody by micro-ELISA was 0.27 (<0.25).
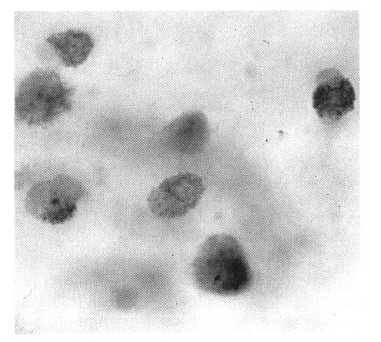
Serial chest radiography (Figure 2A, 2B) and high resolution computed tomography (Figure 3A, 3B, 3C) showed that the pulmonary nodules and patchy densities migrated to other sites and changed in shape and size daily. On the second day of admission, the patient underwent fiberoptic bronchoscopy that revealed no endobronchial lesion. The bronchoalveolar lavage (BAL) fluid had cell proportions of 45% macrophage 15% lymphocytes, 15% neutrophils, and 25% eosinophils (Figure 4). The BAL fluid was negative for bacterial, acid-fast bacilli, and fungi. A transbronchial lung biopsy was performed at the right middle lobe. Microscopy of the lung tissue showed eosinophilic infiltration (Figure 5). Abdominal ultrasound revealed no specific abnormalities except mild splenomegaly. On the ninth day of admission, migrating nodular densities on chest radiographs decreased and then disappeared dramatically following oral praziquantel therapy (Figure 6). Blood eosinophilia consequently decreased and normalized. There was no evidence of recurrence after one year of follow-up.
DISCUSSION
In 1932, Loffler described a syndrome of self-limiting, transient pulmonary infiltrates associated with blood eosinophilia and mild pulmonary symptoms such as cough, malaise, fever, and constitutional symptoms1). Loffler described the associated radiographs as showing migratory shadows that were sometimes homogeneous, but more often spotty or cloudy, of variable delineation (single or multiple, unilateral or bilateral) that disappeared in three to eight days. Radiography of this syndrome typically shows migrating bilateral infiltrates of a non-segmental distribution and fluffy alveolar infiltrates in the upper lobes.
Eosinophilic lung diseases have been given variable names on the basis of etiology, clinical manifestation, and their pathophysiology. For example, Crofton et al. divided them into five catergories, and Reeder and Goodrich used the term pulmonary infiltration with eosinophilia (PIE) syndrome5). In 1969, Liebow and Carrington defined pulmonary infiltrations of the lung by eosinophils, which may or may not be accompanied by an excess of eosinophils in the peripheral blood6). Currently eosinophilic lung diseases are classified or defined by the severity of symptoms, abnormalities on chest radiography, and by the presence and extent of peripheral blood eosinophilia.
Histopathological findings in these cases disclose dense aggregates of eosinophils, histiocytes, and multinuclear giant cells with alveolar spaces, interstitium, and bronchioles with scattered lymphoctes and plasma cells. The pulmonary eosinophilias are a diverse group of disorders whose etiologies include various pulmonary infections (especially parasites, Aspergillus, and mycobacteria), some tumors (HodgkinŌĆÖs lymphomas), drugs, chemicals, pollens, and collagen vascular disorders7).
At the time of presentation, eosinophilic pulmonary infiltrations are initially believed to be atypical community-acquired pneumonias. Most bacterial pneumonias, however, can be virtually ruled out by the presence of increased percentages of eosinophils. Eosinophilia may be noted in Pneumocystis carinii pneumonia, fungal pneumonia, and parasitic infections involving the lung, such as Strongyloides stercoralis, Filaria, Toxocara, Ascaris, Paragonimus, and Schistosoma8).
A number of drugs can also cause eosinophilic lung disease. Drugs reported to cause clinical presentations similar to LofflerŌĆÖs syndrome include nitrofurantoin, phenytoin, L-tryptophan, acetaminophen, ampicillin, heroin, and cocaine. Clinical clues that favor drug-induced pulmonary eosinophilia the presence of rash or blood eosinophilia.
Eosinophils often play an important role not only in host defense mechanisms (due to their lethal effects against helminths, protozoa, fungi, and other microorganisms) but also in various pulmonary infections, some tumors, and collagen vascular diseases.
Two mechanisms of pulmonary eosinophilic infiltrations in parasitic infestations have been postulated in the literature. The first is direct invasion of the lung as in Ascaris, Schistosomes, Filaria, P. westermani, Acylostoma duodenale, necator americanus, Strongyloides, or Echinococcus. Second, allergic reactions to Entameba histolytica, T. trichiura, Toxocar canis or Clonorchis sinensis may be the cause9). In cases such as this one, immunologic stimulation from the life cycle of Clonorchis sinensis in humans may cause the pulmonary infiltrations.
The eosinophilic chemotaxis in parasitic infestations may result from one or more of the following mechanisms : 1) IgE-mediated reactivity against the parasite 2) a direct chemotactic property of certain parasites 3) T cell-dependent mechanisms and 4) immune complex mechanisms. The pulmonary infiltrates themselves may represent a combination of migrating organisms as well as a pulmonary reaction against them. In our patient with migrating pulmonary infiltration, the hypersensitivity response may be the major factor10).
When faced with a patient having signs consistent with eosinophilic pulmonary infiltrations, a differential diagnosis should be included to exclude other disorders such as collagen vascular disease, allergic bronchopulmonary aspergillosis, bronchocentric granulomatosis, sarcoidosis, HodgkinŌĆÖs disease, hypereosinophilic syndrome, helminthic, fungal infection, and drugs.
Infestation with Clonorchis sinensis, or so-called liver fluke, is a very common disease in Korea involving the liver and intestines. Significant amounts of eosinophilia and marked leukocytosis are present in most Koreans infected by Clonorchis sinensis. However, pulmonary infiltrations have been reported in only one case in Korea11). In that case, diffuse bilateral nodular pulmonary infiltrations were the initial chest X-ray finding. But in our case, eosinophilic pneumonia appeared as a solitary pulmonary nodule, with multiple migrating nodules thereafter developing on the other sites.
We can not exactly explain why eosinophilic pulmonary infiltration associated with Clonorchis sinensis is rarely reported despite the higher prevalence of hepatobiliary clonorchiasis in Korea. This could be due to an exceptionally low level of this sort of co-morbidity so low as to be very rarely detected. Alternatively, as the case reported here, the eosinophilic pneumonia due to Clonorchis sinensis could be very transient with few or no symptom that it could elude an examining physician. We suspect that this latter explanation is more likely to be accurate due to the transient, self-limiting, and relatively asymptomatic nature of the pulmonary involvement.
This case enlarges the growing list of etiologic agents in the production of the so-called LofflerŌĆÖs syndrome.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





