 |
 |
| Korean J Intern Med > Volume 38(2); 2023 > Article |
|
Abstract
The incidence of chronic kidney disease (CKD) is increasing worldwide and the current prevalence rate is 13.4%. There are > 120 million CKD patients in China and this number is expected to increase. One of the main abnormalities in patients with CKD and kidney impairment is decreased synthesis of erythropoietin (EPO), which causes anemia and affects iron metabolism. The probability of developing is higher in anemia patients with CKD than in the general population, and the incidence increases as kidney function decreases. Deficient EPO production by the kidney is the most important cause of renal anemia. Notably, anemia in patients with CKD has multiple causes, such as bleeding caused by platelet dysfunction, iron deficiency due to digestive and absorption disorders of the gastrointestinal tract, and shorter red blood cell life. Anemia is also a leading cause of hospitalization in patients with CKD. A new oral medication to treat renal anemia, the hypoxia-inducible factor prolyl hydroxylase inhibitor called roxadustat (FG-4592), regulates iron metabolism and promotes erythropoiesis. This drug has a therapeutic effect on patients with CKD. Roxadustat showed advantages over EPO in clinical experiments. This review summarizes the mechanisms of action, clinical applications, effectiveness, and safety of roxadustat.
Chronic kidney disease (CKD) has diverse causes and affects the health of people worldwide. Approximately 10% of the world’s population suffers from CKD, and this rate is expected to increase in the future [1–3]. Anemia is a serious complication of CKD, and a variety of factors can cause renal anemia (Fig. 1). More than 90% of patients on dialysis have anemia [4], which leads to an increase in morbidity and mortality [5]. Recent studies have shown that transfusion rates decrease, and clinical outcomes improve, in CKD patients when anemia is effectively treated [6]. Renal anemia requires aggressive treatment, as it increases the probability of blood transfusion or hospitalization, and can be fatal [5,7]. Traditional treatments, including iron agents and erythropoiesis-stimulating agents (ESAs), are the main treatment options for renal anemia. Iron replacement is an effective alternative to increase iron stores and hemoglobin levels. However, the safety of long-term intravenous administration of iron agents is unknown [8]. Intravenous medications, such as intravenous iron, are poorly tolerated and have several side effects. Long-term use of ESAs is related to an increased risk of cardiovascular diseases and infections, such that ESAs are a second-line drug treatment for renal anemia [9,10].
Several hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors have been used clinically to improve renal anemia, among which roxadustat, daprodustat, and vadadustat have been best-studied. All three drugs significantly improve renal anemia, but differ in half-life, side effects, and Food and Drug Administration (FDA) approval status; we briefly summarize these differences in Table 1.
Roxadustat is a prolyl hydroxylase inhibitor that increases HIF transcriptional activity by stabilizing HIF-α subunits. The increased transcriptional activity promotes erythropoiesis by activating related enzymes and receptors, including erythropoietin (EPO) and iron-related enzymes and receptors. Many options are available for treating renal anemia, among which promoting hematopoiesis by increasing HIF activity has emerged as a promising new treatment modality. HIF prolyl hydroxylase inhibitors treat anemia in CKD patients by increasing the EPO level and modulating inflammation and iron handling. In particular, these inhibitors decrease the hepcidin level [11]. Prolyl hydroxylase enzymes sense oxygen tension, according to which the HIF level changes [12]. Prolyl hydroxylase enzyme activity decreases with oxygen tension, which causes HIF-α subunit packing and increases HIF transcriptional activity; this leads to an increase in EPO expression, as well as iron recycling and absorption [13]. Some phase 2 trials of other HIF prolyl hydroxylase inhibitors have reported hyperkalemia as a side effect [14,15]. Larger phase 3 trials have provided a more complete picture, indicating that hyperkalemia may be a class effect of HIF prolyl hydroxylase inhibitors. These observations will help guide the design of future trials and clinical monitoring. We have compared the occurrence of hyperkalemia among the different HIF prolyl hydroxylase inhibitors in Table 2 [16–20].
Roxadustat mimics the body’s natural response to hypoxia, thus improving anemia in a reversible manner. Intermittent administration of roxadustat is used to treat anemia [21] in CKD patients and exerts a lasting effect [22–24]. Roxadustat is administered three times per week; the half-life of the drug is 10 hours, which is sufficient to restore HIF transcriptional activity to baseline levels. Hypoxia activates target genes, thereby promoting erythropoiesis during this period [25,26].
Patients starting dialysis in rural areas have much lower hemoglobin levels than those in urban areas, and heart failure and mortality rates are higher [27]. Before 1989, i.e., before the approval of ESAs, anemia in patients with CKD could only be relieved by blood transfusion, and EPO improved their condition. Anemia decreases quality of life, causes left ventricular hypertrophy and increases mortality. ESAs effectively increase the hemoglobin level to the ideal range (9 to 11 g/dL), thus reducing the adverse effects of anemia [28,29]. However, several clinical studies have reported that the efficacy of ESAs is unsatisfactory and they do not improve clinical outcomes; in fact, they can increase the risk of death and stroke [30–32]. Why does normalizing the hemoglobin level not reverse the effects of anemia in patients with CKD? A post hoc analysis showed that higher doses of ESAs were correlated with worse clinical outcomes; however, no clear pathophysiological mechanisms have been established [33,34]; more intensive research is needed to identify the specific mechanisms.
In dialysis patients, roxadustat was not inferior to ESAs [20]; the mean change in hemoglobin levels from baseline to weeks 23 and 27 was in fact greater with roxadustat administration than with ESAs, although the difference was not significant. Unlike ESAs, roxadustat affects iron metabolism, which not only increases transferrin levels but also maintains serum iron levels, thereby preventing any reduction of transferrin saturation. Roxadustat reduces total cholesterol and low-density lipoprotein (LDL) cholesterol more than ESAs. In addition, roxadustat reduces the hepcidin level. Hyperkalemia and upper respiratory tract infections were detected as side effects in the roxadustat group, while hypertension was more common in the ESA group. The same study showed that roxadustat was more likely to cause hyperkalemia than ESAs, which may explain why high doses of ESAs contributed to poor clinical outcomes. Although reports of hyperkalemia may be affected by the bias inherent in open-label trials [35], a double-blind study of nondialysis patients also showed that patients receiving roxadustat were more prone to hyperkalemia and metabolic acidosis compared with those in the ESA group [19]. Global phase 3 trials have been undertaken to provide further data on roxadustat.
The cardiovascular safety of roxadustat was demonstrated in preliminary trials reported by the American Society of Nephrology Kidney Week in 2019, and the incidence rates of hyperkalemia in roxadustat, ESA and placebo groups were similar in these large global trials. Because several clinical experiments have reported an increased probability of developing hyperkalemia when using this agent, caution is needed in patients prone to hyperkalemia. The differences between roxadustat and ESAs are summarized in Table 3 [11,36–39].
Previous studies on roxadustat have focused on hemodialysis (HD) patients. One study of dialysis patients reported that roxadustat was no less effective than ESAs [20]. Roxadustat significantly improved anemia compared with ESAs. In addition, roxadustat affects iron metabolism by increasing transferrin levels and enhancing total iron-binding capacity, as well as stabilizing serum iron levels. These effects may be related to the ability of roxadustat to alter the biomarker levels of iron. Interestingly, roxadustat enhances mean arterial pressure compared with ESAs. HD patients taking roxadustat were more likely to develop hyperkalemia than those taking ESAs. However, that study was limited in terms of sample size and duration; larger and longer international phase 3 studies are therefore required. Nevertheless, this phase 3 trial showed noninferiority of roxadustat compared with ESAs for treating anemia in patients undergoing HD.
Several studies have found that higher ESA doses do not improve the low hemoglobin levels of patients with high C-reactive protein (CRP) levels relative to those with normal levels. This result is consistent with previously published findings of inflammation suppression by ESAs [40,41]. In contrast, in accordance with phase 2 studies of roxadustat, the effect of roxadustat on hemoglobin was not dependent on inflammatory status. Another study that assessed inflammation status according to CRP [23] reported that hemoglobin levels improved independent of the baseline CRP level when patients were in an inflammatory state. The effect of roxadustat on hemoglobin was not related to the baseline CRP level, which explains why roxadustat is effective in CKD patients even if they are in an inflammatory state [42–44]. As such, roxadustat may be an appropriate option for patients in a high inflammatory state with low reactivity to ESAs undergoing HD. Furthermore, it was speculated that the stability of serum iron in the roxadustat group was associated with reduced hepcidin levels, which allows iron absorption by the gut and enhances iron conversion to transferrin in macrophages [45]. However, the influence of ethnicity may preclude extrapolation of some of the experimental results to other populations.
Some new hypotheses have been proposed in the past 2 years in association with deeper research on roxadustat and confirmation of its ability to improve anemia and regulate iron metabolism. Roxadustat reduces LDL cholesterol in CKD dialysis patients compared to ESAs, which is beneficial for the cardiovascular and cerebrovascular systems [46,47]. However, the specific mechanism of the reduction in LDL cholesterol is still unclear and may be related to the expression of an insulin-induced gene [47]. Contrary to the view that roxadustat is more likely to cause hyperkalemia in HD patients than ESAs, Provenzano et al. [48] found a lower probability of hyperkalemia in a roxadustat group compared with an ESA group. However, the serum potassium levels in the roxadustat and ESA groups were comparable during the treatment period in two other studies [46,49]. The details of roxadustat treatment in CKD patients undergoing HD are summarized in Table 4.
A few studies have investigated the effects of roxadustat on peritoneal dialysis (PD) patients, and most of the clinical studies have been reported in China. For example, one experiment included a patient undergoing PD, so that study did not provide statistical support for the efficacy of roxadustat in patients treated with continuous ambulatory PD [50]. In another study of 12 patients undergoing PD, the efficacy of roxadustat was independent of inflammatory status and baseline iron repletion status, and the drug also reduced serum hepcidin levels [44].
In a clinical study of the treatment of anemia in CKD patients undergoing PD, which was the earliest report of roxadustat use in this population [51], oral roxadustat not only increased hemoglobin, but also maintained it within the target range regardless of whether it was directly orally administered without ESA therapy or the patient had been switched from ESA to roxadustat. Furthermore, a decrease in serum hepcidin levels was detected in all of the treatment groups in that study, consistent with the conclusions of previous studies on roxadustat [22,24,44,52]. This finding also suggests increased iron metabolism and absorption [51]. These randomized, phase 3, multicenter, open-label studies have improved our understanding of the efficacy and safety of roxadustat in PD patients, and were followed by similar studies carried out worldwide, particularly in China. In addition to the effects mentioned above, several recent studies of PD patients have shown that roxadustat decreases total cholesterol and LDL [53], and even reduces systolic pressure [54]. Roxadustat has less effect on residual renal function than ESAs. Roxadustat was safe and well-tolerated overall by patients undergoing PD. The most common adverse effects were hyperkalemia, hypertension, insomnia, a hemoglobin level above the target value [50–52], baseline electrocardiogram abnormalities, transient arteriovenous block, and transient liver function abnormalities [44]. Also, some patients experience itching, although it is believed that this is not caused by roxadustat. Two patients reportedly developed severe hypertension, but this was resolved via dose reduction of roxadustat [55].
Few studies have been conducted on the efficacy of roxadustat in anemic patients undergoing PD, and the reference value of current studies is limited. Therefore, larger multicenter, long-term clinical studies are needed to provide more definitive conclusions. The details of roxadustat treatment in CKD patients undergoing PD are summarized in Table 5 [44,53,54,56].
Roxadustat increases hemoglobin levels in nondialysis patients with CKD regardless of intravenous iron administration [23,51]. Roxadustat significantly increases hemoglobin levels in patients diagnosed with CKD and is more effective than placebo in patients who are not on dialysis, and noninferior to ESAs in patients undergoing HD. Roxadustat is an effective treatment option for these patients; however, due to limitations in sample size and study length, the efficacy of roxadustat requires further exploration [19,20]. Similar to dialysis patients, roxadustat significantly improved anemia status and reduced hepcidin and total cholesterol levels. Hepcidin increases iron transport protein synthesis and iron absorption by the gut, which is downregulated by hypoxia and the stabilization of HIF. Hepcidin plays a key regulatory role in iron mobilization and absorption from macrophages and hepatocytes [57,58]. The mechanism underlying the cholesterol-lowering effect of roxadustat is unclear, but it may be related to acetyl coenzyme A, which is required for the first step in cholesterol synthesis and degradation of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase [59,60].
Statins reduce the risk of cardiovascular disease in CKD patients who are not on dialysis [61]; if roxadustat can lower cholesterol, it may benefit cardiovascular vessels. However, similar to dialysis patients, hyperkalemia and metabolic acidosis remain common adverse reactions of roxadustat in patients not undergoing dialysis. A clinical study performed in China confirmed the efficacy of roxadustat for treating anemia and maintaining hemoglobin [19], but the effects of the drug on iron metabolism may have been underestimated [62]. A systematic review and meta-analysis of dialysis and nondialysis patients reported that roxadustat significantly increased hemoglobin levels, and reduced those of hepcidin and ferritin, compared with placebo and ESAs. Transferrin levels also improved, but this was more pronounced in nondialysis patients [63].
In the past 2 years, clinical studies of roxadustat in nondialysis patients have shown that, in addition to improving renal anemia and reducing LDL cholesterol and hepcidin, roxadustat also decreases the need for blood transfusion and intravenous iron supplementation compared with ESAs and placebo [64–67]. Similar to dialysis patients, the efficacy of roxadustat for improving anemia was not affected by the inflammatory status of nondialysis patients, in contrast to ESAs [65,66]. Overall, the safety of roxadustat was similar to that of ESAs and placebo. More common adverse events include end-stage kidney disease, pneumonia, hypertension, and urinary tract infection [65]. The details of roxadustat treatment in nondialysis CKD patients are summarized in Table 6.
Roxadustat is excreted in the feces and urine. Some scholars believe that CKD affects the nonrenal clearance of roxadustat [50] via the accumulation of uremic toxins, which leads to changes in the expression or activity of drug-metabolizing enzymes and transporters [68,69]. The effective t1/2 of roxadustat is a suitable parameter to predict drug accumulation better than the t1/2 because it has multicompartmental kinetics [45]. Furthermore, HD/hemodiafiltration does not affect the clearance of roxadustat, suggesting that roxadustat can be administered before or after dialysis [50].
Despite the remarkable efficacy of roxadustat in the treatment of renal anemia, the safety concerns cannot be ignored. However, previous clinical studies revealed no difference in treatment-emergent adverse effects (TEAEs) between roxadustat and control or placebo groups. The most frequent adverse effects of patients in the roxadustat group were thromboembolism, hypertension, insomnia, hemoglobin levels exceeding the target, peripheral edema, hyperphosphatemia, dyspnea, seizure, and nausea. Furthermore, the probability of treatment discontinuation because of TEAEs was higher in the roxadustat group [46].
Potassium levels vary among clinical studies. Chen et al. [19,20] reported that the most common adverse effects in their roxadustat group were hyperkalemia and metabolic acidosis. We speculate that this is related to the intracellular hypoxia-like environment caused by the HIF-stabilizing effect of roxadustat. Roxadustat as a HIF prolyl hydroxylase inhibitor modulates the switch from aerobic to anaerobic metabolism. The consequence of increased glycolysis is tissue acidification associated with the overproduction of lactic acid. Acidosis causes potassium to move from the inside to outside of the cell, which leads to hyperkalemia [62]. However, several recent clinical studies have drawn different conclusions regarding the effect of roxadustat on serum potassium. No difference in serum potassium distribution was detected between the two groups in some studies [46,49,66], while hyperkalemia was higher in the ESA than roxadustat group in some clinical trials [48,65]. The effect of roxadustat on serum potassium is not mentioned in the drug instructions. Based on the results of clinical studies, hyperkalemia is considered an off-target rather than class effect of roxadustat.
In August 2021, an FDA advisory committee reported that while roxadustat was comparable to ESAs in terms of efficacy, it posed a risk of severe thromboembolic events, among other adverse effects. Recent clinical studies also showed that roxadustat posed a higher risk of thromboembolic events than placebo and ESAs, regardless of dialysis status, such as arteriovenous fistula thrombosis, deep vein thrombosis, pulmonary embolism, and arteriovenous access thrombosis [45,46,48,64,67]. A meta-analysis published in 2022 also noted that roxadustat poses a higher risk of deep venous thrombosis in nondialysis-dependent CKD patients [70]. However, these studies did not explain the mechanism underlying the thromboembolism risk. According to an exploratory analysis by the FDA advisory committee, greater increases and decreases in hemoglobin were associated with higher rates of thromboembolic events. The developers of roxadustat speculate that thromboembolic risk may be lowered by using a lower starting dose, although this remains to be confirmed. As mentioned above, a lower starting dose of roxadustat in PD patient achieves the target hemoglobin level more effectively than the standard dose [56]. Therefore, in our next study we will explore the relationship between a lower starting dose of roxadustat and thromboembolic risk. Furthermore, roxadustat increases vascular endothelial growth factor (VEGF) levels; this raises concerns about its effects on tumors because VEGF has effects on angiogenesis, vascular permeability, and tumor formation [71]. However, transcription of the VEGF gene is regulated by HIF-1α and HIF-2α binding to hypoxia response elements [72].
Roxadustat is a new drug to treat renal anemia that increases hemoglobin and serum transferrin levels, as well as intestinal iron absorption, and also reduces the level of hepcidin. Moreover, it is effective in both nondialysis-dependent and dialysis-dependent CKD patients. Roxadustat improves patient compliance, but the long-term safety and efficacy require further study; thus, larger prospective multicenter clinical studies are needed.
Acknowledgments
This study was supported by funding from the National Natural Science Foundation of China (82000703); Science and technology development fund of Affiliated Hospital of Xuzhou Medical University (XYFY2020038). We are sincerely grateful to every member of the research team and to our hospital staff for their support and contribution.
Figure 1
Etiology and treatment options for renal anemia. ESA, erythropoiesis-stimulating agents; HIF, hypoxia-inducible factor.
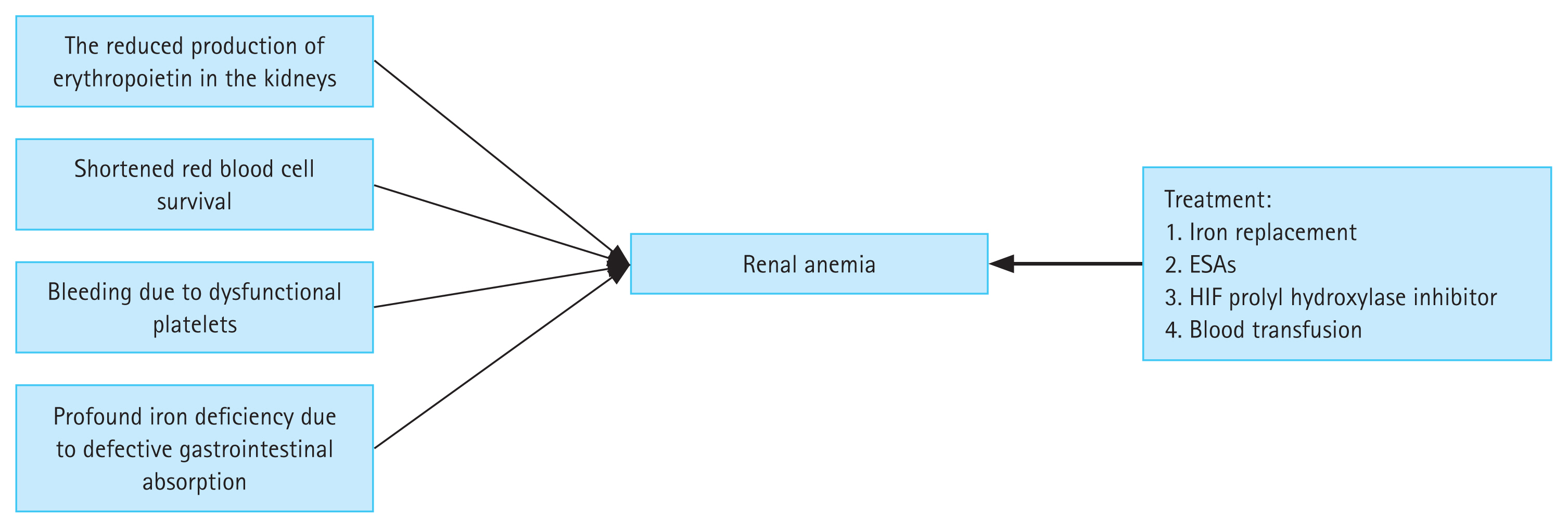
Table 1
Differences among roxadustat, daprodustat, and vadadustat
Table 2
Hyperkalemia in patients treated with different hypoxia-inducible factor prolyl hydroxylase inhibitors
| Type | Incidence of hyperkalemia |
|---|---|
| Daprodustat | Patients who were not undergoing dialysis, daprodustat was not associated with an increased risk of hyperkalemia [16]. |
| Desidustat and molidustat | Trials of desidustat and molidustat did not show an increased risk of hyperkalemia [17,18]. |
| Roxadustat | Hyperkalemia and metabolic acidosis occurred more frequently with roxadustat versus the comparator groups [19,20]. |
Table 3
Differences between roxadustat and erythropoiesis-stimulating agents
| Type | Roxadustat | ESAs |
|---|---|---|
| Mechanism of action | By activating the body’s natural response to hypoxia, independent of cellular oxygen levels [11,36]. | ESAs act directly on erythroid progenitor cells, thus to play the hematopoietic function. |
| Method of drug administration | Orally administered treatment | Administered parenterally |
| Treatment effect | Roxadustat maintained hemoglobin within 10–12 g/dL in patients on hemodialysis and was noninferior to darbepoetin alfa [37]. | The means of hemoglobin levels were similar in the two treatment groups (roxadustat and ESAs) [37]. |
| Limitations | The drug is expensive for most patients with CKD. | Their efficacy is reduced in patients with inflammation [38]. |
| Treatment-emergent adverse events | The incidences of nasopharyngitis and vomiting were higher in the roxadustat group than in the ESAs group. | Current evidence suggest ESAs are associated with increased risks of thromboembolic events increased when given at a higher than recommended dose [39]. |
| Treatment compliance | The mean treatment compliance was high in both treatment groups (roxadustat, 99.23%) [37]. | ESAs, 100% [37] |
Table 4
Details of roxadustat treatment in patients with chronic kidney disease undergoing hemodialysis
| Type | Roxadustat |
|---|---|
| Treatment effect | The roxadustat group significantly improved anemia compared with the ESAs group. |
| Side effects | Roxadustat was more likely to develop hyperkalemia in hemodialysis patients than ESAs. But in several other studies, the effect of roxalostat on blood potassium was not uniform [46,48,49]. |
| Interesting phenomenon | Roxadustat not only has an effect on iron metabolism but also improves mean arterial pressure, in addition, the effect of roxadustat in improving anemia was unaffected by the inflammatory state. |
| Research status | Overall, efficacy studies of roxadustat have mainly focused on HD patients, but larger clinical experiments with longer study cycles are urgently needed. |
Table 5
Details of roxadustat treatment in patients with chronic kidney disease undergoing peritoneal dialysis
| Type | Roxadustat |
|---|---|
| Treatment effect | Roxadustat not only improves anemia, increases iron metabolism and absorption, but also lowers total cholesterol and LDL, and even reduces systolic pressure. |
| Side effects | Roxadustat was overall safe and well-tolerated. The most common adverse effects were hyperkalemia, hypertension, insomnia, hemoglobin level above target [44,53,54]. |
| Interesting phenomenon | For PD patients, the lower starting dose of roxadustat also achieves the therapeutic target of hemoglobin compared with the standard dose [56]. |
| Research status | In general, there are few studies on the efficacy of roxadustat about anemia in patients with PD, so a few current studies can only provide us with limited reference value. |
Table 6
Details of roxadustat treatment in patients with chronic kidney disease not undergoing dialysis
REFERENCES
1. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–822.


2. Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014;9:e84943.



3. Smith RE Jr.. The clinical and economic burden of anemia. Am J Manag Care 2010;16:Suppl Issues. S59–S66.

4. Souaid T, Taliercio J, Simon JF, Mehdi A, Nakhoul GN. Anemia of chronic kidney disease: will new agents deliver on their promise? Cleve Clin J Med 2022;89:212–222.


5. Horl WH. Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol 2013;9:291–301.



6. Benz R, Schmidt R, Kelly K, Wolfson M. Epoetin alfa once every 2 weeks is effective for initiation of treatment of anemia of chronic kidney disease. Clin J Am Soc Nephrol 2007;2:215–221.


7. Seliger S, Fox KM, Gandra SR, et al. Timing of erythropoiesis-stimulating agent initiation and adverse outcomes in nondialysis CKD: a propensity-matched observational study. Clin J Am Soc Nephrol 2010;5:882–888.



8. Agarwal R, Kusek JW, Pappas MK. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 2015;88:905–914.



9. Macdougall IC, White C, Anker SD, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 2019;380:447–458.


10. Sakaguchi Y, Hamano T, Wada A, Masakane I. Types of erythropoietin-stimulating agents and mortality among patients undergoing hemodialysis. J Am Soc Nephrol 2019;30:1037–1048.



11. Kaplan JM, Sharma N, Dikdan S. Hypoxia-inducible factor and its role in the management of anemia in chronic kidney disease. Int J Mol Sci 2018;19:389.



12. Semenza GL, Agani F, Booth G, et al. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int 1997;51:553–555.


13. Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle 2008;7:28–32.


14. Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int 2016;90:1115–1122.


15. Meadowcroft AM, Cizman B, Holdstock L, et al. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J 2019;12:139–148.



16. Holdstock L, Cizman B, Meadowcroft AM, et al. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants with chronic kidney disease. Clin Kidney J 2019;12:129–138.



17. Akizawa T, Macdougall IC, Berns JS, et al. Long-term efficacy and safety of molidustat for anemia in chronic kidney disease: DIALOGUE Extension Studies. Am J Nephrol 2019;49:271–280.




18. Parmar DV, Kansagra KA, Patel JC, et al. Outcomes of desidustat treatment in people with anemia and chronic kidney disease: a phase 2 study. Am J Nephrol 2019;49:470–478.



19. Chen N, Hao C, Peng X, et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med 2019;381:1001–1010.


20. Chen N, Hao C, Liu BC, et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med 2019;381:1011–1022.


21. Nangaku M, Kojima I, Tanaka T, Ohse T, Kato H, Fujita T. Novel drugs and the response to hypoxia: HIF stabilizers and prolyl hydroxylase. Recent Pat Cardiovasc Drug Discov 2006;1:129–139.


22. Provenzano R, Besarab A, Wright S, et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis 2016;67:912–924.


23. Provenzano R, Besarab A, Sun CH, et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol 2016;11:982–991.



24. Besarab A, Provenzano R, Hertel J, et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 2015;30:1665–1673.



25. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 1995;92:5510–5514.



26. Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 1998;8:588–594.


27. Zhang W, Gong Z, Peng X, Tang S, Bi M, Huang W. Clinical characteristics and outcomes of rural patients with ESRD in Guangxi, China: one dialysis center experience. Int Urol Nephrol 2010;42:195–204.



28. Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease: results of a phase III multicenter clinical trial. Ann Intern Med 1989;111:992–1000.


29. Mocks J. Cardiovascular mortality in haemodialysis patients treated with epoetin beta: a retrospective study. Nephron 2000;86:455–462.



30. Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006;355:2071–2084.


31. Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006;355:2085–2098.


32. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009;361:2019–2032.


33. Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 2008;74:791–798.


34. Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 2010;363:1146–1155.


35. Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586.



36. Locatelli F, Fishbane S, Block GA, Macdougall IC. Targeting hypoxia-inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol 2017;45:187–199.



37. Akizawa T, Iwasaki M, Yamaguchi Y, Majikawa Y, Reusch M. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol 2020;31:1628–1639.



38. Johnson DW, Pollock CA, Macdougall IC. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology (Carlton) 2007;12:321–330.

39. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol 2008;26:132–149.


40. Gomes AC, Gomes MS. Hematopoietic niches, erythropoiesis and anemia of chronic infection. Exp Hematol 2016;44:85–91.


41. Bradbury BD, Critchlow CW, Weir MR, Stewart R, Krishnan M, Hakim RH. Impact of elevated C-reactive protein levels on erythropoiesis-stimulating agent (ESA) dose and responsiveness in hemodialysis patients. Nephrol Dial Transplant 2009;24:919–925.


42. Amparo FC, Kamimura MA, Molnar MZ, et al. Diagnostic validation and prognostic significance of the Malnutrition-Inflammation Score in nondialyzed chronic kidney disease patients. Nephrol Dial Transplant 2015;30:821–828.


43. Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 2004;65:1009–1016.


44. Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol 2016;27:1225–1233.



46. Fishbane S, Pollock CA, El-Shahawy M, et al. Roxadustat versus epoetin alfa for treating anemia in patients with chronic kidney disease on dialysis: results from the randomized phase 3 ROCKIES study. J Am Soc Nephrol 2022;33:850–866.


47. Csiky B, Schomig M, Esposito C, et al. Roxadustat for the maintenance treatment of anemia in patients with end-stage kidney disease on stable dialysis: a European phase 3, randomized, open-label, active-controlled study (PYRENEES). Adv Ther 2021;38:5361–5380.




48. Provenzano R, Shutov E, Eremeeva L, et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant 2021;36:1717–1730.



49. Charytan C, Manllo-Karim R, Martin ER, et al. A randomized trial of roxadustat in anemia of kidney failure: SIERRAS study. Kidney Int Rep 2021;6:1829–1839.



50. Groenendaal-van de Meent D, Kerbusch V, Kaspera R, et al. Effect of kidney function and dialysis on the pharmacokinetics and pharmacodynamics of roxadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor. Eur J Drug Metab Pharmacokinet 2021;46:141–153.




51. Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open-label study. Ther Apher Dial 2020;24:115–125.




52. Chen N, Qian J, Chen J, et al. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant 2017;32:1373–1386.



53. Hou YP, Mao XY, Wang C, et al. Roxadustat treatment for anemia in peritoneal dialysis patients: a randomized controlled trial. J Formos Med Assoc 2022;121:529–538.


54. Wu T, Qi Y, Ma S, et al. Efficacy of roxadustat on anemia and residual renal function in patients new to peritoneal dialysis. Ren Fail 2022;44:529–540.



55. Chen J, Li Z, Zhang H, et al. A prospective, self-controlled pilot study of the efficacy of roxadustat for erythropoietin hyporesponsiveness in patients requiring chronic ambulatory peritoneal dialysis. J Ren Nutr 2022;32:595–604.


56. Yang Z, Ma T, Xu X, et al. Randomized study on the efficacy of standard versus low roxadustat dose for anemia in patients on peritoneal dialysis. Kidney Int Rep 2021;7:455–464.



58. Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest 2007;117:1926–1932.



59. Nguyen AD, McDonald JG, Bruick RK, DeBose-Boyd RA. Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J Biol Chem 2007;282:27436–27446.


60. Hwang S, Nguyen AD, Jo Y, Engelking LJ, Brugarolas J, De-Bose-Boyd RA. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem 2017;292:9382–9393.



61. Ku E, Campese V. Is lipid management effective for all stages of CKD? Blood Purif 2013;35:26–30.



62. Anker MS, Butler J, Anker SD. Roxadustat for anemia in patients with chronic kidney disease. N Engl J Med 2020;383:e3.


63. Liu J, Zhang A, Hayden JC, et al. Roxadustat (FG-4592) treatment for anemia in dialysis-dependent (DD) and not dialysis-dependent (NDD) chronic kidney disease patients: a systematic review and meta-analysis. Pharmacol Res 2020;155:104747.


64. Shutov E, Sulowicz W, Esposito C, et al. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, double-blind, placebo-controlled study (ALPS). Nephrol Dial Transplant 2021;36:1629–1639.




65. Fishbane S, El-Shahawy MA, Pecoits-Filho R, et al. Roxadustat for treating anemia in patients with CKD not on dialysis: results from a randomized phase 3 study. J Am Soc Nephrol 2021;32:737–755.



66. Coyne DW, Roger SD, Shin SK, et al. Roxadustat for CKD-related anemia in non-dialysis patients. Kidney Int Rep 2020;6:624–635.



67. Barratt J, Andric B, Tataradze A, et al. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, open-label, active-controlled study (DOLOMITES). Nephrol Dial Transplant 2021;36:1616–1628.




68. Rowland Yeo K, Aarabi M, Jamei M, Rostami-Hodjegan A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev Clin Pharmacol 2011;4:261–274.


69. Nolin TD, Frye RF, Matzke GR. Hepatic drug metabolism and transport in patients with kidney disease. Am J Kidney Dis 2003;42:906–925.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



